Informatics Educational Institutions & Programs
Contents
| |
| Names | |
|---|---|
| Preferred IUPAC name
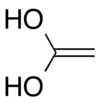
Ethene-1,1-diol | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H4O2 | |
| Molar mass | 60.052 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1,1-Dihydroxyethene is an organic compound consisting of two hydroxy groups as substituents on the same carbon atom of an ethene chain. The chemical is also called ketene hydrate because it is the carbonyl hydrate of ketene. Its structure can also be considered as the enol form of acetic acid. This compound is likely a key intermediate in the hydration reaction that converts ketene into acetic acid.[1] The analysis of the possible pathways for this reaction has been cited as an example of the importance of considering activation energy of each mechanistic step.[2] The hydration of the carbonyl of ketene, which formally involves an addition reaction of one water molecule onto the carbonyl group, is likely catalyzed by a second water molecule.[3] The compound has now been synthesized and identified spectroscopically.[4]
References
- ^ Nguyen, Minh Tho; Raspoet, Greet (1999). "The hydration mechanism of ketene: 15 years later". Can. J. Chem. 77 (5–6): 817–829. doi:10.1139/v99-090.
- ^ Guthrie, J. Peter (2011). "No barrier theory and the origins of the intrinsic barrier". In Richard, John P. (ed.). Advances in Physical Organic Chemistry. Vol. 45. Academic Press. pp. 205–208. ISBN 9780123860477.
- ^ Nguyen, Thanh Lam; Xue, Bert C.; Ellison, G. Barney; Stanton, John F. (2013). "Theoretical Study of Reaction of Ketene with Water in the Gas Phase: Formation of Acetic Acid?". J. Phys. Chem. A. 117 (43): 10997–11005. Bibcode:2013JPCA..11710997N. doi:10.1021/jp408337y. PMID 24087932.
- ^ Mardyukov, Artur; Eckhardt, André K.; Schreiner, Peter R. (2020). "1,1-Ethenediol: The Long Elusive Enol of Acetic Acid". Angew. Chem. Int. Ed. 59 (14): 5577–5580. doi:10.1002/anie.201915646. PMC 7154680. PMID 31899845.

















