Informatics Educational Institutions & Programs
Contents

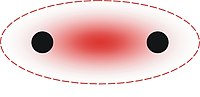
In chemistry, aurophilicity refers to the tendency of gold complexes to aggregate via formation of weak metallophilic interactions.[1][2]
The main evidence for aurophilicity is from the crystallographic analysis of Au(I) complexes. The aurophilic bond has a length of about 3.0 Å and a strength of about 7–12 kcal/mol,[1] which is comparable to the strength of a hydrogen bond. The effect is greatest for gold as compared with copper or silver—the higher elements in its periodic table group—due to increased relativistic effects.[1][3] Observations and theory show that, on average, 28% of the binding energy in the aurophilic interaction can be attributed to relativistic expansion of the gold d orbitals.[4]
An example of aurophilicity is the propensity of gold centres to aggregate. While both intramolecular and intermolecular aurophilic interactions have been observed, only intramolecular aggregation has been observed at such nucleation sites.[5]
Role in self-assembly

The similarity in strength between hydrogen bonding and aurophilic interaction has proven to be a convenient tool in the field of polymer chemistry. Much research has been conducted on self-assembling supramolecular structures, both those that aggregate by aurophilicity alone and those that contain both aurophilic and hydrogen-bonding interactions.[6] An important and exploitable property of aurophilic interactions relevant to their supramolecular chemistry is that while both inter- and intramolecular interactions are possible, intermolecular aurophilic linkages are comparatively weak and easily broken by solvation; most complexes that exhibit intramolecular aurophilic interactions retain such moieties in solution.[1]
References
- ^ a b c d e f Schmidbaur, Hubert (2000). "The Aurophilicity Phenomenon: A Decade of Experimental Findings, Theoretical Concepts and Emerging Application". Gold Bulletin. 33 (1): 3–10. doi:10.1007/BF03215477.
- ^ Schmidbaur, Hubert (1995). "Ludwig Mond Lecture: High-Carat Gold Compounds". Chem. Soc. Rev. 24 (6): 391–400. doi:10.1039/CS9952400391.
- ^ Behnam Assadollahzadeh & Peter Schwerdtfeger (2008). "A comparison of metallophilic interactions in group 11[X–M–PH3]n (n = 2–3) complex halides (M = Cu, Ag, Au; X = Cl, Br, I) from density functional theory". Chemical Physics Letters. 462 (4–6): 222–228. Bibcode:2008CPL...462..222A. doi:10.1016/j.cplett.2008.07.096.
- ^ Nino Runeberg; Martin Schütz & Hans-Joachim Werner (1999). "The aurophilic attraction as interpreted by local correlation methods". J. Chem. Phys. 110 (15): 7210–7215. Bibcode:1999JChPh.110.7210R. doi:10.1063/1.478665.
- ^ Hubert Schmidbaur; Stephanie Cronje; Bratislav Djordjevic & Oliver Schuster (2005). "Understanding gold chemistry through relativity". J. Chem. Phys. 311 (1–2): 151–161. Bibcode:2005CP....311..151S. doi:10.1016/j.chemphys.2004.09.023.
- ^ William J. Hunks; Michael C. Jennings & Richard J. Puddephatt (2002). "Supramolecular Gold(I) Thiobarbiturate Chemistry: Combining Aurophilicity and Hydrogen Bonding to Make Polymers, Sheets, and Networks". Inorg. Chem. 41 (17): 4590–4598. doi:10.1021/ic020178h.