Informatics Educational Institutions & Programs
Contents

| |
| |
| Names | |
|---|---|
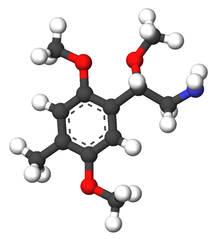
| Preferred IUPAC name
2-(2,5-Dimethoxy-4-methylphenyl)-2-methoxyethan-1-amine | |
| Other names
4-Methyl-2,5,β-trimethoxyphenethylamine
2-(4-Methyl-2,5,β-trimethoxyphenyl)ethanamine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H19NO3 | |
| Molar mass | 225.288 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
BOD (4-methyl-2,5,β-trimethoxyphenethylamine) is a lesser-known psychedelic drug. It is the beta-methoxy analog of 2C-D and was first synthesized by Alexander Shulgin. In his book PiHKAL, the dosage range is listed as 15–25 mg with a duration of 8–16 hours.[1] Its reported effects include mild open-eye and moderate closed-eye alterations in visual perception, enhancement of conversation and sense of humor, and unpleasant physical effects such as nausea and lethargy.[2] Very little data exists about the pharmacological properties, metabolism, and toxicity of BOD.
Legality
United States
BOD is unscheduled in the United States, but purchase, sale, or possession for human consumption could be prosecuted under the Federal Analogue Act.[3]
United Kingdom
This substance is a Class A drug in the Drugs controlled by the UK Misuse of Drugs Act.[4]
See also
References
- ^ BOD Entry in PiHKAL
- ^ Shulgin, Alexander; Shulgin, Ann (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628.
- ^ "21 U.S. Code § 841 - Prohibited acts A", LII / Legal Information Institute, retrieved 2016-08-02
- ^ "UK Misuse of Drugs act 2001 Amendment summary". Isomer Design. Retrieved 12 March 2014.

















