Informatics Educational Institutions & Programs
Contents
| |
| Names | |
|---|---|
| Preferred IUPAC name
Cyclooctadeca-1,3,5,7,9,11,13,15,17-nonayne | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C18 | |
| Molar mass | 216.198 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
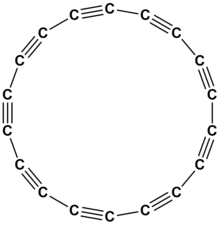
Cyclooctadeca-1,3,5,7,9,11,13,15,17-nonayne or cyclo[18]carbon is an allotrope of carbon with molecular formula C
18. The molecule is a ring of eighteen carbon atoms, connected by alternating triple and single bonds; thus, it is a polyyne and a cyclocarbon.
Cyclo[18]carbon is the smallest cyclo[n]carbon predicted to be thermodynamically stable, with a computed strain energy of 72 kilocalories per mole.[1] Above 122 K, it explosively decomposes to amorphous graphite.[2]
A collaboration of teams at IBM and the University of Oxford team claimed to synthesize it in solid state in 2019[3] by electrochemical decarbonylation of several sites of a cyclobutanone structure:[4] Later, researchers from Spain have used computational techniques to probe the structural and electronic properties of the molecule, and have discovered it to be an electron acceptor.[5]

According to these IBM researchers, the electronic structure of their product consists of alternating triple bonds and single bonds, rather than a cumulene-type structure of consecutive double bonds. This supposedly makes this molecule a semiconductor.[4]
References
- ^ George A. Adamson; Charles W. Rees (1996). "Towards the total synthesis of cyclo[n]carbons and the generation of cyclo[6]carbon". J. Chem. Soc., Perkin Trans. 1 (13): 1535–1543. doi:10.1039/P19960001535.
- ^ François Diederich; Yves Rubin; Carolyn B. Knobler; Robert L. Whetten; Kenneth E. Schriver; Kendall N. Houk; Yi Li (8 September 1989). "All-Carbon Molecules: Evidence for the Generation of Cyclo[18]carbon from a Stable Organic Precursor". Science. 245 (4922): 1088–1090. Bibcode:1989Sci...245.1088D. doi:10.1126/science.245.4922.1088. PMID 17838807. S2CID 23726682.
- ^ Kaiser, Katharina; Scriven, Lorel M.; Schulz, Fabian; Gawel, Przemyslaw; Gross, Leo; Anderson, Harry L. (15 Aug 2019). "An sp-hybridized molecular carbon allotrope, cyclo[18]carbon". Science. 365 (6459): 1299–1301. arXiv:1908.05904. doi:10.1126/science.aay1914. PMID 31416933. S2CID 201019470.
- ^ a b Castelvecchi, Davide (15 August 2019). "Chemists make first-ever ring of pure carbon". Nature. 572 (7770): 426. doi:10.1038/d41586-019-02473-z. PMID 31431741.
- ^ Stasyuk, Anton J.; Stasyuk, Olga A.; Solà, Miquel; Voityuk, Alexander A. (29 Nov 2019). "Cyclo[18]carbon: the smallest all-carbon electron acceptor". Chemical Communications. 56 (3): 352–355. doi:10.1039/c9cc08399e. hdl:10256/17613. PMID 31825030. S2CID 209316603.

















