Informatics Educational Institutions & Programs
Contents
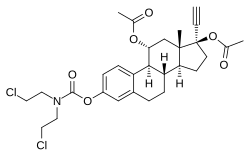 | |
| Clinical data | |
|---|---|
| Other names | 11α-Hydroxyethinylestradiol 3-(bis(2-chloroethyl)carbamate) 11α,17β-diacetate; 17α-Ethynylestra-1,3,5(10)-triene-3,11α,17β-triol 11α,17β-diacetate 3-(bis(2-chloroethyl)carbamate) |
| Identifiers | |
| |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C29H35Cl2NO6 |
| Molar mass | 564.50 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Cytestrol acetate is a steroidal antiestrogen and a cytostatic antineoplastic agent (i.e., chemotherapeutic) which was developed for the treatment of breast cancer but was never marketed.[1][2][3][4]
It is an 11α-hydroxylated derivative of ethinylestradiol in which a bis(2-chloroethyl)amine nitrogen mustard moiety has been attached as an ester at the C3 position and acetate esters have been attached at the C11α and C17β positions.[1][2][3][4] The mechanism of action of cytestrol acetate in breast cancer is two-fold: (1) acting as an antiestrogen similarly to fulvestrant or ICI-164384; and (2) having cytostatic actions via the carbamate–nitrogen mustard moiety analogously to estramustine phosphate.[1][2][3][4] The drug shows potent efficacy against breast cancer superior to that of tamoxifen in in vitro models.[1][2][3]
See also
References
- ^ a b c d Oborotov AV, Smirnova ZS, Osetrova IP, Polozkova AP, Rzheznikov VM (1999). "Antitumor activity of various medicinal forms of the new estrogenocytostatic drug cytestrol acetate". Pharmaceutical Chemistry Journal. 33 (10): 526–527. doi:10.1007/BF02508372. ISSN 0091-150X. S2CID 5550495.
- ^ a b c d Smirnova ZS, Rzheznikov VM, Tolkachev VN, Borisova LM, Kiseleva MP, Semeĭkin AV, et al. (2014). "[Antitumor and antiproliferative action of the steroidal cytostatic antiestrogen cytestrol acetate on hormone-dependent tumor models]". Eksperimental'naia i Klinicheskaia Farmakologiia (in Russian). 77 (10): 31–35. PMID 25518525.
- ^ a b c d Smirnova ZS (2003). "[Experimental Study of Hormonocytostatics for Treatment of Breast Cancer.]" [Russian biotherapeutic journal]. Российский биотерапевтический журнал (in Russian). 2 (2).
- ^ a b c Smirnova ZS, Rzheznikov VM, Tolkachev VN, Borisova LM, Kiseleva MP, Semeykin AV, Fedocheva TA, Shirokikh KE, Banin VV, Shimanovsky NL (November 2014). "Противоопухолевое и антипролиферативное действие стероидного антиэстрогена цитэстрола ацетата на моделях гормонозависимых опухолей" [Antitumor and antiproliferative effects of the steroid antiestrogen citestrol acetate in models of hormone-dependent tumors.]. Экспериментальная и клиническая фармакология [Experimental and clinical pharmacology.] (in Russian). 77 (10): 31–35.

















