Informatics Educational Institutions & Programs
Contents
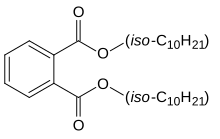
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Bis(8-methylnonyl) benzene-1,2-dicarboxylate | |
| Other names
Bis(8-methylnonyl) phthalate
Bis(isodecyl) phthalate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.043.601 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C28H46O4 | |
| Molar mass | 446.672 g·mol−1 |
| Density | 0.96-0.97 g/cm3 at 20 °C[1] |
| Melting point | −50 °C (−58 °F; 223 K)[1] |
| Boiling point | 250 to 257 °C (482 to 495 °F; 523 to 530 K) at 0.5 kPa [2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Diisodecyl phthalate (DIDP) is a commonly used plasticizer used in the production of plastic and plastic coating to increase flexibility. It is a mixture of compounds derived from the esterification of phthalic acid and isomeric decyl alcohols.
The coating on furnishings, cookware, pharmaceutical pills, food wrappers and many other products may have DIDP or other phthalates in them. There has been recent concern in the US and European Union for their toxicity and bioaccumulative quality. The European Union has set a maximum specific migration limit (SML) from food contact materials of 9 mg/kg food for the sum of diisodecyl phthalates and diisononyl phthalates.[3]
DIDP has been listed since 2007 under Proposition 65 as a substance known to the state of California to cause reproductive toxicity.[4] The similar compound DINP is also listed.
In 2013, ECHA's Risk Assessment Committee (RAC) concluded that Di-isodecyl phthalate (DIDP) does not warrant classification for reprotoxic effects under the EU's Classification, Labelling and Packaging (CLP) regulation.[5]
See also
- DPHP, a similar phthalate ester
References
- ^ a b Record of CAS RN 26761-40-0 in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on Sep 27, 2007.
- ^ NIOSH. "International Chemical Safety Cards". NIOSH. Archived from the original on 2017-06-14. Retrieved 2017-09-09.
- ^ "EU legislative list for food contact materials".
- ^ "OEHHA Proposition 65 List of Chemicals".
- ^ "Evaluation of new scientific evidence concerning DINP and DIDP". European Chemicals Agency. Archived from the original on 2022-09-01. Retrieved 2020-01-26.

















