Informatics Educational Institutions & Programs
Contents
| |
| |
| Names | |
|---|---|
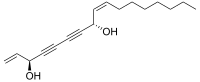
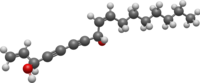
| Preferred IUPAC name
(3R,8S,9Z)-Heptadeca-1,9-diene-4,6-diyne-3,8-diol | |
| Other names
cis-Heptadeca-1,9-diene-4,6-diyne-3,8-diol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C17H24O2 | |
| Molar mass | 260.377 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Falcarindiol is a polyyne found in carrot roots which has antifungal activity.[1][2] Falcarindiol is the main compound responsible for bitterness in carrots.[3] Falcarindiol and other falcarindiol-type polyacetylenes are also found in many other plants of the family Apiaceae, including some commonly used seasonings such as dill and parsley.[4]
A variety of bioactivities have been reported for falcaridiol and the falcarindiol-type polyacetylenes,[5][6][7] and because of potential health-promoting metabolic effects these compounds are studied as potential nutraceuticals.[8] Falcarindiol is the most-active among several polyynes found in Devil's club (Oplopanax horridus) that inhibit cell proliferation.[9]
See also
References
- ^ Garrod, B. (1978). "Cis-heptadeca-1,9-diene-4,6-diyne-3,8-diol, an antifungal polyacetylene from carrot root tissue". Physiological Plant Pathology. 13 (2): 241–246. doi:10.1016/0048-4059(78)90039-5.
- ^ Kemp, M. S. (1978). "Falcarindiol: An antifungal polyacetylene from Aegopodium podagraria". Phytochemistry. 17 (5): 1002. Bibcode:1978PChem..17.1002K. doi:10.1016/S0031-9422(00)88669-0.
- ^ Czepa, A.; Hofmann, T. (2003). "Structural and sensory characterization of compounds contributing to the bitter off-taste of carrots (Daucus carota L.) and carrot puree". Journal of Agricultural and Food Chemistry. 51 (13): 3865–3873. doi:10.1021/jf034085+. PMID 12797757.
- ^ Christensen, L. P.; Brandt, K. (2006). "Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis". Journal of Pharmaceutical and Biomedical Analysis. 41 (3): 683–693. doi:10.1016/j.jpba.2006.01.057. PMID 16520011.
- ^ Jin, H. R.; Zhao, J.; Zhang, Z.; Liao, Y.; Wang, C. Z.; Huang, W. H.; Li, S. P.; He, T. C.; Yuan, C. S.; Du, W. (2012). "The antitumor natural compound falcarindiol promotes cancer cell death by inducing endoplasmic reticulum stress". Cell Death and Disease. 3 (8): e376. doi:10.1038/cddis.2012.122. PMC 3434669. PMID 22914324.
- ^ Wyrembek, P.; Negri, R.; Kaczor, P.; Czyżewska, M.; Appendino, G.; Mozrzymas, J. W. (2012). "Falcarindiol allosterically modulates GABAergic currents in cultured rat hippocampal neurons". Journal of Natural Products. 75 (4): 610–616. doi:10.1021/np2008522. PMID 22432736.
- ^ Wang, Limei; Palme, Veronika; Schilcher, Nicole; Ladurner, Angela; Heiss, Elke H.; Stangl, Herbert; Bauer, Rudolf; Dirsch, Verena M.; Atanasov, Atanas G. (2017). "The Dietary Constituent Falcarindiol Promotes Cholesterol Efflux from THP-1 Macrophages by Increasing ABCA1 Gene Transcription and Protein Stability". Frontiers in Pharmacology. 8: 596. doi:10.3389/fphar.2017.00596. PMC 5585181. PMID 28919859..
- ^ Christensen, L. P. (2011). "Aliphatic C17-Polyacetylenes of the Falcarinol Type as Potential Health Promoting Compounds in Food Plants of the Apiaceae Family". Recent Patents on Food, Nutrition & Agriculture. 3 (1): 64–77. doi:10.2174/2212798411103010064. PMID 21114468.
- ^ Sun, S; Du, GJ; Qi, LW; Williams, S; Wang, CZ; Yuan, CS (2010). "Hydrophobic constituents and their potential anticancer activities from Devil's Club (Oplopanax horridus Miq.)". J. Ethnopharmacol. 132 (1): 280–285. doi:10.1016/j.jep.2010.08.026. PMC 3050531. PMID 20723598.

















