Informatics Educational Institutions & Programs
Contents

| Part of a series of articles on |
| Nanomaterials |
|---|
 |
| Carbon nanotubes |
| Fullerenes |
| Other nanoparticles |
| Nanostructured materials |
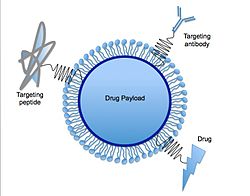
Lipid nanoparticles (LNPs) are nanoparticles composed of lipids. They are a novel pharmaceutical drug delivery system (and part of nanoparticle drug delivery), and a novel pharmaceutical formulation.[1][2] LNPs as a drug delivery vehicle were first approved in 2018 for the siRNA drug Onpattro.[3] LNPs became more widely known in late 2020, as some COVID-19 vaccines that use RNA vaccine technology coat the fragile mRNA strands with PEGylated lipid nanoparticles as their delivery vehicle (including both the Moderna and the Pfizer–BioNTech COVID-19 vaccines).[4]
Characteristics
A lipid nanoparticle is typically spherical with an average diameter between 10 and 1000 nanometers. Solid lipid nanoparticles possess a solid lipid core matrix that can solubilize lipophilic molecules. The lipid core is stabilized by surfactants (emulsifiers). The emulsifier used depends on administration routes and is more limited for parenteral administrations.[5] The term lipid is used here in a broader sense and includes triglycerides (e.g. tristearin), diglycerides (e.g. glycerol bahenate), monoglycerides (e.g. glycerol monostearate), fatty acids (e.g. stearic acid), steroids (e.g. cholesterol), and waxes (e.g. cetyl palmitate). All classes of emulsifiers (with respect to charge and molecular weight) have been used to stabilize the lipid dispersion. It has been found that the combination of emulsifiers might prevent particle agglomeration more efficiently.[5][6]
An SLN is generally spherical in shape and consists of a solid lipid core stabilized by a surfactant. The core lipids can be fatty acids, acylglycerols, waxes, and mixtures of these surfactants. Biological membrane lipids such as phospholipids, sphingomyelins, bile salts (sodium taurocholate), and sterols (cholesterol) are utilized as stabilizers. Biological lipids having minimum carrier cytotoxicity and the solid state of the lipid permit better controlled drug release due to increased mass transfer resistance.[7] Shah et al. in their book Lipid Nanoparticles: Production, Characterization and Stability discuss these in detail.[citation needed]
LNPs used in mRNA vaccines for SARS-CoV-2 (the virus that causes COVID-19) are made of four types of lipids: an ionizable cationic lipid (whose positive charge binds to negatively charged mRNA), a PEGylated lipid (for stability), a phospholipid (for structure), and cholesterol (for structure).[8] Because of rapid clearance by the immune system of the positively charged lipid, neutral ionizable amino lipids were developed. A novel squaramide lipid (that is, partially aromatic four-membered rings, which can participate in pi–pi interactions) has been a favored part of the delivery system used, for example, by Moderna.[9]
Synthesis
Different formulation procedures include high shear homogenization and ultrasound, solvent emulsification/evaporation, or microemulsion. Obtaining size distributions in the range of 30-180 nm is possible using ultrasonification at the cost of long sonication time. Solvent-emulsification is suitable in preparing small, homogeneously sized lipid nanoparticles dispersions with the advantage of avoiding heat.[10]
The obtained LNP formulation can subsequently be filled into sterile containers and subjected to final quality control. However, various measures to monitor and evaluate product quality are integrated in every step of LNP manufacturing and include testing of polydispersity, particle size, drug loading efficiency and endotoxin levels.[11]
Applications
Development of solid lipid nanoparticles is one of the emerging fields of lipid nanotechnology (for a review on lipid nanotechnology, see [12]) with several potential applications in drug delivery, clinical medicine and research, as well as in other disciplines. Due to their unique size-dependent properties, lipid nanoparticles offer the possibility to develop new therapeutics. The ability to incorporate drugs into nanocarriers offers a new prototype in drug delivery that could hold great promise for attaining the bioavailability enhancement along with controlled and site-specific drug delivery. SLN's are also considered to well tolerated in general, due to their composition from physiologically similar lipids.[citation needed]
The conventional approaches such as use of permeation enhancers, surface modification, prodrug synthesis, complex formation and colloidal lipid carrier-based strategies have been developed for the delivery of drugs to intestinal lymphatics. In addition, polymeric nanoparticles, self-emulsifying delivery systems, liposomes, microemulsions, micellar solutions and recently solid lipid nanoparticles (SLN) have been exploited as probable possibilities as carriers for oral intestinal lymphatic delivery.[13]
Drug delivery
Solid lipid nanoparticles can function as the basis for oral and parenteral drug delivery systems. SLNs combine the advantages of lipid emulsion and polymeric nanoparticle systems while overcoming the temporal and in vivo stability issues that troubles the conventional as well as polymeric nanoparticles drug delivery approaches.[5] It has been proposed that SLNs combine numerous advantages over the other colloidal carriers i.e. incorporation of lipophilic and hydrophilic drugs feasible, no biotoxicity of the carrier, avoidance of organic solvents, possibility of controlled drug release and drug targeting, increased drug stability and no problems with respect to large scale production.[5] Furthermore, various functions such as molecules for targeting, PEG chains for stealth properties[14] or thiol groups for adhesion via disulfide bond formation[15] can be immobilized on their surface. A recent study has demonstrated the use of solid lipid nanoparticles as a platform for oral delivery of the nutrient mineral iron, by incorporating the hydrophilic molecule ferrous sulfate (FeSO4) in a lipid matrix composed of stearic acid.[16] Carvedilol-loaded solid lipid nanoparticles were prepared using hot-homogenization technique for oral delivery using compritol and poloxamer 188 as a lipid and surfactant, respectively.[17] Another example of drug delivery using SLN would be oral solid SLN suspended in distilled water, which was synthesized to trap drugs within the SLN structure. Upon indigestion, the SLNs are exposed to gastric and intestinal acids that dissolve the SLNs and release the drugs into the system.[18]
Many nano-structured systems have been employed for ocular drug delivery and yielded some promising results. SLNs have been looked at as a potential drug carrier system since the 1990s. SLNs do not show biotoxicity as they are prepared from physiological lipids. SLNs are especially useful in ocular drug delivery as they can enhance the corneal absorption of drugs and improve the ocular bioavailability of both hydrophilic and lipophilic drugs.[19] Solid lipid nanoparticles have another advantage of allowing autoclave sterilization, a necessary step towards formulation of ocular preparations.[20]
Advantages of SLNs include the use of physiological lipids (which decreases the danger of acute and chronic toxicity), the avoidance of organic solvents, a potential wide application spectrum (dermal, per os, intravenous) and the high pressure homogenization as an established production method. Additionally, improved bioavailability, protection of sensitive drug molecules from the outer environment water, light) and even controlled release characteristics were claimed by the incorporation of poorly water-soluble drugs in the solid lipid matrix. Moreover, SLN can carry both lipophilic and hydrophilic drugs and are more affordable compared to polymeric/surfactant-based carriers.[21]
Nucleic acids
A significant obstacle to using LNPs as a delivery vehicle for nucleic acids is that in nature, lipids and nucleic acids both carry a negative electric charge—meaning they do not easily mix with each other.[22] While working at Syntex in the mid-1980s,[23] Philip Felgner pioneered the use of artificially-created cationic lipids (positively-charged lipids) to bind lipids to nucleic acids in order to transfect the latter into cells.[24] However, by the late 1990s, it was known from in vitro experiments that this use of cationic lipids had undesired side effects on cell membranes.[25]
During the late 1990s and 2000s, Pieter Cullis of the University of British Columbia developed ionizable cationic lipids which are "positively charged at an acidic pH but neutral in the blood."[8] Cullis also led the development of a technique involving careful adjustments to pH during the process of mixing ingredients in order to create LNPs which could safely pass through the cell membranes of living organisms.[22][26] As of 2021, the current understanding of LNPs formulated with such ionizable cationic lipids is that they enter cells through receptor-mediated endocytosis and end up inside endosomes.[8] The acidity inside the endosomes causes LNPs' ionizable cationic lipids to acquire a positive charge, and this is thought to allow LNPs to escape from endosomes and release their RNA payloads.[8]
From 2005 into the early 2010s, LNPs were investigated as a drug delivery system for small interfering RNA (siRNA) drugs.[8] In 2009, Cullis co-founded a company called Acuitas Therapeutics to commercialize his LNP research; Acuitas worked on developing LNPs for Alnylam Pharmaceuticals's siRNA drugs.[27] In 2018, the FDA approved Alnylam's siRNA drug Onpattro (patisiran), the first drug to use LNPs as the drug delivery system.[3][8]
By that point in time, siRNA drug developers like Alnylam were already looking at other options for future drugs like chemical conjugate systems, but during the 2010s, the earlier research into using LNPs for siRNA became a foundation for new research into using LNPs for mRNA.[8] Lipids intended for short siRNA strands did not work well for much longer mRNA strands, which led to extensive research during the mid-2010s into the creation of novel ionizable cationic lipids appropriate for mRNA.[8] As of late 2020, several mRNA vaccines for SARS-CoV-2 use LNPs as their drug delivery system, including both the Moderna COVID-19 vaccine and the Pfizer–BioNTech COVID-19 vaccines.[3] Moderna uses its own proprietary ionizable cationic lipid called SM-102, while Pfizer and BioNTech licensed an ionizable cationic lipid called ALC-0315 from Acuitas.[8]
Lymphatic absorption mechanism
Elucidation of intestinal lymphatic absorption mechanism from solid lipid nanoparticles using Caco-2 cell line as in vitro model was developed.[28] Several researchers have shown the enhancement of oral bioavailibility of poorly water-soluble drugs when encapsulated in solid lipid nanoparticle. This enhanced bioavailibility is achieved via lymphatic delivery. To elucidate the absorption mechanism, from solid lipid nanoparticle, human excised Caco-2 cell monolayer could be alternative tissue for development of an in-vitro model to be used as a screening tool before animal studies are undertaken. The results obtained in this model suggested that the main absorption mechanism of carvedilol loaded solid lipid nanoparticle could be endocytosis and, more specifically, clathrin-mediated endocytosis. [17]
See also
- Nanomedicine, the general field
- Micelle, lipid cored
- Liposome, lipid bilayer shell, an earlier form with some limitations
- Lipoplex, a complex of plasmid or linear DNA and lipids
- Targeted drug delivery
- mRNA-1273, from Moderna, uses LNPs
- BNT162b2, from BioNTech/Pfizer, uses LNPs
References
- ^ Saupe, Anne; Rades, Thomas (2006). "Solid Lipid Nanoparticles". Nanocarrier Technologies. pp. 41–50. doi:10.1007/978-1-4020-5041-1_3. ISBN 978-1-4020-5040-4.
- ^ Jenning, V; Thünemann, AF; Gohla, SH (2000). "Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids". International Journal of Pharmaceutics. 199 (2): 167–77. doi:10.1016/S0378-5173(00)00378-1. PMID 10802410.
- ^ a b c Cooney, Elizabeth (1 December 2020). "How nanotechnology helps mRNA Covid-19 vaccines work". Stat. Retrieved 3 December 2020.
- ^ Pardi, Norbert; Hogan, Michael J.; Porter, Frederick W.; Weissman, Drew (April 2018). "mRNA vaccines — a new era in vaccinology". Nature Reviews Drug Discovery. 17 (4): 261–279. doi:10.1038/nrd.2017.243. PMC 5906799. PMID 29326426.
- ^ a b c d Mehnert et al., 2001
- ^ Small, 1986
- ^ Manzunath et al., 2005
- ^ a b c d e f g h i Cross, Ryan (March 6, 2021). "Without these lipid shells, there would be no mRNA vaccines for COVID-19". Chemical & Engineering News. American Chemical Society. Retrieved March 6, 2021.
- ^ Cornebise, Mark; Narayanan, Elisabeth; Xia, Yan (November 12, 2021). "Discovery of a Novel Amino Lipid That Improves Lipid Nanoparticle Performance through Specific Interactions with mRNA". Advanced Functional Materials. 32 (8). Wiley: 2106727. doi:10.1002/adfm.202106727. S2CID 244085785.
- ^ Wolfgang Mehnert, Karsten Mäder, Solid lipid nanoparticles: Production, characterization and applications, Advanced Drug Delivery Reviews, Volume 64, 2012, Pages 83-101, ISSN 0169-409X, https://doi.org/10.1016/j.addr.2012.09.021
- ^ Marciniak, Mike (June 21, 2023). "Lipid nanoparticle (LNP) manufacturing: Challenges & Solutions". Retrieved July 5, 2023.
- ^ Mashaghi, S.; Jadidi, T.; Koenderink, G.; Mashaghi, A. Lipid Nanotechnology. Int. J. Mol. Sci. 2013, 14, 4242-4282.[1]
- ^ Studies on binary lipid matrix-based solid lipid nanoparticles of repaglinide: in vitro and in vivo evaluation. Rawat MK, Jain A and Singh S, Journal of Pharmaceutical Sciences, 2011, volume 100, issue 6, pages 2366-2378
- ^ Fam, SY; Chee, CF; Yong, CY; Ho, KL; Mariatulqabtiah, AR; Tan, WS (April 2020). "Stealth Coating of Nanoparticles in Drug-Delivery Systems". Nanomaterials. 10 (4): 787. doi:10.3390/nano10040787. PMC 7221919. PMID 32325941.
- ^ Hock, N; Racaniello, GF; Aspinall, S; Denora, N; Khutoryanskiy, V; Bernkop-Schnürch, A (2022). "Thiolated Nanoparticles for Biomedical Applications: Mimicking the Workhorses of our Body". Adv Sci (Weinh). 9 (1): 2102451. doi:10.1002/advs.202102451. PMC 8728822. PMID 34773391.
- ^ Zariwala, MG (November 2013). "A novel approach to oral iron delivery using ferrous sulphate loaded solid lipid nanoparticles" (PDF). Int J Pharm. 456 (2): 400–7. doi:10.1016/j.ijpharm.2013.08.070. PMID 24012860.
- ^ a b Shah, Mansi K.; Madan, Parshotam; Lin, Senshang (23 May 2013). "Preparation, evaluation and statistical optimization of carvedilol-loaded solid lipid nanoparticle for lymphatic absorption via oral administration". Pharmaceutical Development and Technology. 19 (4): 475–485. doi:10.3109/10837450.2013.795169. PMID 23697916. S2CID 42174732.
- ^ Pandey, Rajesh; Sharma, Sadhna; Khuller, G.K. (2005). "Oral solid lipid nanoparticle-based antitubercular chemotherapy". Tuberculosis. 85 (5–6): 415–420. doi:10.1016/j.tube.2005.08.009. PMID 16256437.
- ^ Arana, Lide; Salado, Clarisa; Vega, Sandra; Aizpurua-Olaizola, Oier; Arada, Igor de la; Suarez, Tatiana; Usobiaga, Aresatz; Arrondo, José Luis R.; Alonso, Alicia (2015-11-01). "Solid lipid nanoparticles for delivery of Calendula officinalis extract". Colloids and Surfaces B: Biointerfaces. 135: 18–26. doi:10.1016/j.colsurfb.2015.07.020. PMID 26231862. S2CID 41621205.
- ^ Seyfoddin, Ali; J. Shaw; R. Al-Kassas (2010). "Solid lipid nanoparticles for ocular drug delivery". Drug Delivery. 17 (7): 467–489. doi:10.3109/10717544.2010.483257. PMID 20491540. S2CID 25357639.
- ^ Mukherjee, S et al. “Solid lipid nanoparticles: a modern formulation approach in drug delivery system.” Indian journal of pharmaceutical sciences vol. 71,4 (2009): 349-58. doi:10.4103/0250-474X.57282
- ^ a b Foley, Katherine Ellen (22 December 2020). "The first Covid-19 vaccines have changed biotech forever". Quartz. Quartz Media. Retrieved 11 January 2021.
- ^ Jones, Mark (22 July 1997). "Phil Felgner Interview – July 22, 1997". UC San Diego Library: San Diego Technology Archive. Regents of the University of California.
- ^ Byk, Gerardo (2002). "Cationic lipid-based gene delivery". In Mahato, Ram I.; Kim, Sung Wan (eds.). Pharmaceutical Perspectives of Nucleic Acid-Based Therapeutics. London: Taylor & Francis. pp. 273–303. ISBN 9780203300961.
- ^ Lasic, Danilo D. (1997). Liposomes in Gene Delivery. Boca Raton: CRC Press. p. 191. ISBN 9780849331091. Retrieved 11 January 2021.
- ^ Cullis, Pieter R.; Hope, Michael J. (5 July 2017). "Lipid Nanoparticle Systems for Enabling Gene Therapies". Molecular Therapy. 25 (7): 1467–1475. doi:10.1016/j.ymthe.2017.03.013. PMC 5498813. PMID 28412170.
- ^ Shore, Randy (November 17, 2020). "COVID-19: Vancouver's Acuitas Therapeutics a key contributor to coronavirus solution". Vancouver Sun.
- ^ Shah, Mansi K.; Madan, Parshotam; Lin, Senshang (29 July 2014). "Elucidation of intestinal absorption mechanism of carvedilol-loaded solid lipid nanoparticle using Caco-2 cell line as an model". Pharmaceutical Development and Technology. 20 (7): 877–885. doi:10.3109/10837450.2014.938857. PMID 25069593. S2CID 40506806.
Further reading
- Müller, Rainer H.; Mäder, Karsten; Gohla, Sven (3 July 2000). "Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art". European Journal of Pharmaceutics and Biopharmaceutics. 50 (1): 161–177. doi:10.1016/S0939-6411(00)00087-4. PMID 10840199.
- Shah, Mansi K.; Madan, Parshotam; Lin, Senshang (June 2014). "Preparation, in vitro evaluation and statistical optimization of carvedilol-loaded solid lipid nanoparticles for lymphatic absorption via oral administration". Pharmaceutical Development and Technology. 19 (4): 475–485. doi:10.3109/10837450.2013.795169. PMID 23697916. S2CID 42174732.
- Shah, Mansi K.; Madan, Parshotam; Lin, Senshang (3 October 2015). "Elucidation of intestinal absorption mechanism of carvedilol-loaded solid lipid nanoparticles using Caco-2 cell line as an in-vitro model". Pharmaceutical Development and Technology. 20 (7): 877–885. doi:10.3109/10837450.2014.938857. PMID 25069593. S2CID 40506806.

















