Informatics Educational Institutions & Programs
Contents

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
4,7-Dihydro-1H-tricyclopenta[def,jkl,pqr]triphenylene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H12 | |
| Molar mass | 264.32 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sumanene is a polycyclic aromatic hydrocarbon and of scientific interest because the molecule can be considered a fragment of buckminsterfullerene. Suman means "sunflower" in both Hindi and Sanskrit.[1] The core of the arene is a benzene ring and the periphery consists of alternating benzene rings (3) and cyclopentadiene rings (3). Unlike fullerene, sumanene has benzyl positions which are available for organic reactions.
Organic synthesis
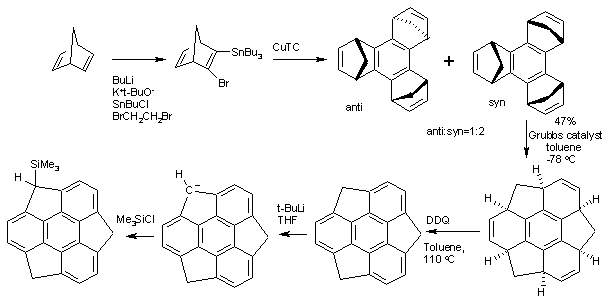
The structure of Sumanene can be inferred from oxidation of 1,5,9-trimethyltriphenylene but the first practical synthesis starts from norbornadiene.[2] Norbornadiene is converted into a stannane by action of n-butyllithium, dibromoethane and tributyltinchloride. An Ullmann reaction of this stannane with CuTC affords the benzene core. The methylene bridges (-CH
2-) created in this conversion then migrate in a tandem ring-opening metathesis and ring-closing metathesis by the Grubbs' catalyst. The final structure is obtained by oxidation by DDQ.
Properties
Sumanene is a bowl-shaped molecule with a bowl depth of 118 picometers.[3] The 6 hub carbon atoms are pyramidalized by 9° and the molecule displays considerable bond alternation (138.1 to 143.1 pm). Sumanene also experiences bowl-to-bowl inversion with an inversion barrier of 19.6 kcal/mol (82 kJ/mol) at 140 °C which is much higher than that found for its corannulene cousin. Like any benzylic proton, the sumanene protons can be abstracted by a strong base such as t-butyl lithium to form the sumanene mono carbanion. This strong nucleophile can react with an electrophile such as trimethylsilyl chloride to the trimethylsilyl derivative.
The trianion has also been reported.[4] Electron transport properties have been investigated [5][6] as well as carbon NMR[7]
Derivatives
Sumanene derivatives [8] such as naphtosumanene [9] and trisialsumanene [10][11] have been described. Chiral sumanenes are of some interest with respect to inherent chirality,[12] examples are chiral trimethylsumanene [13] and a chiral sumanene cyclopentadienyl iron complex [14]
References
- ^ Towards the design of tricyclopenta [def, jkl, pqr] triphenylene (sumanene): a bowl-shaped hydrocarbon featuring a structural motif present in C60 (buckminsterfullerene) Goverdhan Mehta, Shailesh R. Shah and K. Ravikumar Journal of the Chemical Society, Chemical Communications, 1993, (12), 1006 - 1008 doi:10.1039/C39930001006
- ^ A Synthesis of Sumanene, a Fullerene Fragment Hidehiro Sakurai, Taro Daiko, and Toshikazu Hirao Science, Vol 301, Issue 5641, 1878 , 26 September 2003 doi:10.1126/science.1088290
- ^ Structural Elucidation of Sumanene and Generation of Its Benzylic Anions Hidehiro Sakurai, Taro Daiko, Hiroyuki Sakane, Toru Amaya, and Toshikazu Hirao J. Am. Chem. Soc., 127 (33), 11580 -11581, 2005 doi:10.1021/ja0518169
- ^ Hidehiro Sakurai, Taro Daiko, Hiroyuki Sakane, Toru Amaya, and Toshikazu Hira; J. Am. Chem. Soc., 2005, 127 (33), pp 11580–11581 doi:10.1021/ja0518169
- ^ Anisotropic Electron Transport Properties in Sumanene Crystal Toru Amaya, Shu Seki, Toshiyuki Moriuchi, Kana Nakamoto, Takuto Nakata, Hiroyuki Sakane, Akinori Saeki, Seiichi Tagawa and Toshikazu Hirao J. Am. Chem. Soc., 2009, 131 (2), pp 408–409 doi:10.1021/ja805997v
- ^ The electrochemical inspection of the redox activity of sumanene and its concave CpFe complex Piero Zanello, Serena Fedi, Fabrizia Fabrizi de Biani, Gianluca Giorgi, Toru Amaya, Hiroyuki Sakane and Toshikazu Hirao Dalton Trans., 2009, 9192-9197 doi:10.1039/B910711H
- ^ Solid-state 13C NMR investigations of 4,7-dihydro-1H-tricyclopenta[def,jkl,pqr]triphenylene (sumanene) and indeno[1,2,3-cd]fluoranthene: Buckminsterfullerene moieties Merrill D. Halling, Anita M. Orendt, Mark Strohmeier, Mark S. Solum, Vikki M. Tsefrikas, Toshikazu Hirao, Lawrence T. Scott, Ronald J. Pugmire and David M. Grant Phys. Chem. Chem. Phys., 2010, 12, 7934-7941 doi:10.1039/C001903H
- ^ Bowl-to-bowl inversion of sumanene derivatives Toru Amaya, Hiroyuki Sakane, Toshiko Muneishi and Toshikazu Hirao Chem. Commun., 2008, 765-767 doi:10.1039/B712839H
- ^ Synthesis of Highly Strained π-Bowls from Sumanene Toru Amaya, Takuto Nakata and Toshikazu Hirao, Japan J. Am. Chem. Soc., 2009, 131 (31), pp 10810–10811 doi:10.1021/ja9031693
- ^ Communication Development of a Sila-Friedel−Crafts Reaction and Its Application to the Synthesis of Dibenzosilole Derivatives Shunsuke Furukawa, Junji Kobayashi and Takayuki Kawashima J. Am. Chem. Soc., 2009, 131 (40), pp 14192–14193 doi:10.1021/ja906566r
- ^ Synthesis, structures and optical properties of trisilasumanene and its related compounds Tomoharu Tanikawa, Masaichi Saito, Jing Dong Guo and Shigeru Nagase Org. Biomol. Chem., 2011, 9, 1731-1735 doi:10.1039/C0OB00987C
- ^ Inherently chiral concave molecules—from synthesis to applications Agnieszka Szumna Chem. Soc. Rev., 2010, 39, 4274-4285 doi:10.1039/B919527K
- ^ Asymmetric Synthesis of a Chiral Buckybowl, Trimethylsumanene Shuhei Higashibayashi and Hidehiro Sakurai J. Am. Chem. Soc., 2008, 130 (27), pp 8592–8593 doi:10.1021/ja802822k
- ^ Sakane, H., Amaya, T., Moriuchi, T. and Hirao, T. (2009), A Chiral Concave-Bound Cyclopentadienyl Iron Complex of Sumanene. Angewandte Chemie International Edition, 48: 1640–1643. doi:10.1002/anie.200805567