Informatics Educational Institutions & Programs
Contents
| |
| Names | |
|---|---|
| IUPAC name
Phosphanylidyneyttrium
| |
| Other names
Yttrium phosphide, yttrium(III) phosphide.
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.032.318 |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| PY | |
| Molar mass | 119.879600 |
| Appearance | Colourless solid |
| Density | 4.35 g/cm3[1] |
| Melting point | 200.78 °C (393.40 °F; 473.93 K) |
| Boiling point | 511.30 °C (952.34 °F; 784.45 K) |
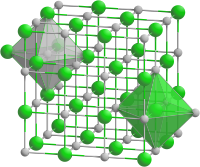
| Structure[2] | |
| Rock salt structure | |
| Fm3m | |
a = 0.5661 nm
| |
Formula units (Z)
|
4 |
| Octahedral at Y3+, Octahedral at P3- | |
| Related compounds | |
Other anions
|
Yttrium nitride Yttrium(III) arsenide Yttrium(III) antimonide |
Other cations
|
Scandium phosphide Lutetium phosphide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Yttrium phosphide is an inorganic compound of yttrium and phosphorus with the chemical formula YP.[3][4][5] The compound may be also classified as yttrium(III) phosphide.
Synthesis
Heating (500–1000 °C) of pure substances in a vacuum:[6]
- 4 Y + P4 → 4 YP
Properties
Yttrium phosphide forms cubic crystals.[1]
Uses
Ytttium phosphide is a semiconductor used in laser diodes, and in high power and frequency applications.
References
- ^ a b "mp-994: YP (cubic, Fm-3m, 225)". materialsproject.org. Retrieved 8 December 2021.
- ^ Parthé, E. (1963-01-01). "Note on the structure of ScP and YP". Acta Crystallographica. 16 (1). International Union of Crystallography (IUCr): 71. Bibcode:1963AcCry..16...71P. doi:10.1107/s0365110x63000141. ISSN 0365-110X.
- ^ "Yttrium Phosphide". American Elements. Retrieved 8 December 2021.
- ^ "Substance Name: Yttrium phosphide (YP)". TOXNET. chem.nlm.nih.gov. Retrieved 15 June 2017.
- ^ "Yttrium: yttrium phosphide". Webelements. webelements.com. Retrieved 15 June 2017.
- ^ Parthé, E. (10 January 1963). "Note on the structure of ScP and YP". Acta Crystallographica. 16: 71. Bibcode:1963AcCry..16...71P. doi:10.1107/S0365110X63000141. Retrieved 12 December 2021.

















