| Approaches to medical decision-making based on big clinical data 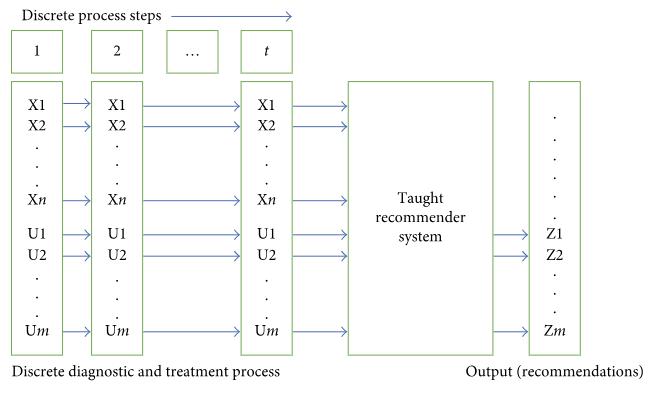 This paper by Malykh and Rudetskiy "discusses different approaches to building a clinical decision support system based on big data," with a focus on non-biased processing methods and their comparative assessments. After an in-depth analysis of methods and objectives, the authors present their findings from the clinical decision support data and their significance. They conclude that case-based and precedent-based approaches each have their advantages--including more accurate recommendations and faster system speeds--but are not without disadvantages. The authors suggest future research is needed to address "problems with optimization of provided metrics, compression of state descriptions, and construction of training procedures." This paper by Malykh and Rudetskiy "discusses different approaches to building a clinical decision support system based on big data," with a focus on non-biased processing methods and their comparative assessments. After an in-depth analysis of methods and objectives, the authors present their findings from the clinical decision support data and their significance. They conclude that case-based and precedent-based approaches each have their advantages--including more accurate recommendations and faster system speeds--but are not without disadvantages. The authors suggest future research is needed to address "problems with optimization of provided metrics, compression of state descriptions, and construction of training procedures." |