 | | November 10, 2020
Laboratory Informatics Weekly Update | Volume 18, Issue 45 | | | | Past, Present, and Future of Cannabis Laboratory Testing and Regulation in the United States  | | 11/10/2020 - Top 3 STARLIMS Features You Probably Aren’t Using, But Should!  Congrats on implementing STARLIMS for your laboratory! This may come as a shock, but you probably aren’t using some of the great features of the LIMS, right out of the box. Like most laboratories, just getting an informatics system in place can be a tough process, forcing a lab to focus on critical items first, like sample reception, testing, and approvals. This means that other enhancements, modules, and features can be placed on the backburner or even forgotten. [Read More] Congrats on implementing STARLIMS for your laboratory! This may come as a shock, but you probably aren’t using some of the great features of the LIMS, right out of the box. Like most laboratories, just getting an informatics system in place can be a tough process, forcing a lab to focus on critical items first, like sample reception, testing, and approvals. This means that other enhancements, modules, and features can be placed on the backburner or even forgotten. [Read More]
11/10/2020 - Integrated Reporting for Anatomic Pathology & Molecular Testing  In today’s anatomic pathology (AP) lab, molecular testing plays an increasingly important role in informing treatment decisions. With Sunquest’s integrated reporting capabilities for AP and molecular testing, review combined results in one actionable report for more coordinated, efficient care. [READ MORE] In today’s anatomic pathology (AP) lab, molecular testing plays an increasingly important role in informing treatment decisions. With Sunquest’s integrated reporting capabilities for AP and molecular testing, review combined results in one actionable report for more coordinated, efficient care. [READ MORE]
11/10/2020 - Strategies for Creating Effective Technical Documentation – Part 1  On April 20th, 2010, a massive explosion on BP’s Macondo well in the Gulf of Mexico killed 11 people and began the largest marine oil spill in history. Before the spill was over, 206 million gallons of oil were released into the Gulf of Mexico, ultimately affecting wildlife and water-quality along hundreds of miles of Gulf coastline. According to BP’s Deepwater Horizon Accident Investigation Report, reasons leading to the accident were lack of good technical documentation and non-adherence to the documents that did exist. [Read More] On April 20th, 2010, a massive explosion on BP’s Macondo well in the Gulf of Mexico killed 11 people and began the largest marine oil spill in history. Before the spill was over, 206 million gallons of oil were released into the Gulf of Mexico, ultimately affecting wildlife and water-quality along hundreds of miles of Gulf coastline. According to BP’s Deepwater Horizon Accident Investigation Report, reasons leading to the accident were lack of good technical documentation and non-adherence to the documents that did exist. [Read More]
11/10/2020 - Exemplar LIMS - Baylor College of Medicine Case Study See how Baylor College of Medicine’s Human Genome Sequencing Center uses Exemplar NGS LIMS from Sapio Sciences The BCM-HGSC is a world leader in genomics and one of the most technologically advanced laboratories processing a high volume and wide variety of samples and protocols. They have experienced dramatic growth in both the volume and complexity of the DNA sequencing including for COVID-19 testing. Click here to see how Baylor uses Exemplar’s NGS LIMS to meet their demand and how Exemplar has become an essential part of their future
11/10/2020 - Autoscribe White Paper Emphasizes the Role of LIMS in Supporting ISO17025 Accreditation in Efficiently Run Laboratories Autoscribe Informatics, a leading global laboratory informatics provider, announced the release of a new white paper examining the role of a Laboratory Information Management System (LIMS) in supporting ISO17025 accreditation. The white paper explores the ways in which a Laboratory Information Management System (LIMS) can play a key role in achieving, maintaining, and benefiting from ISO17025 accreditation.
11/05/2020 - Sample Tracking is Essential for Organizations that Outsource Laboratory Testing Activities 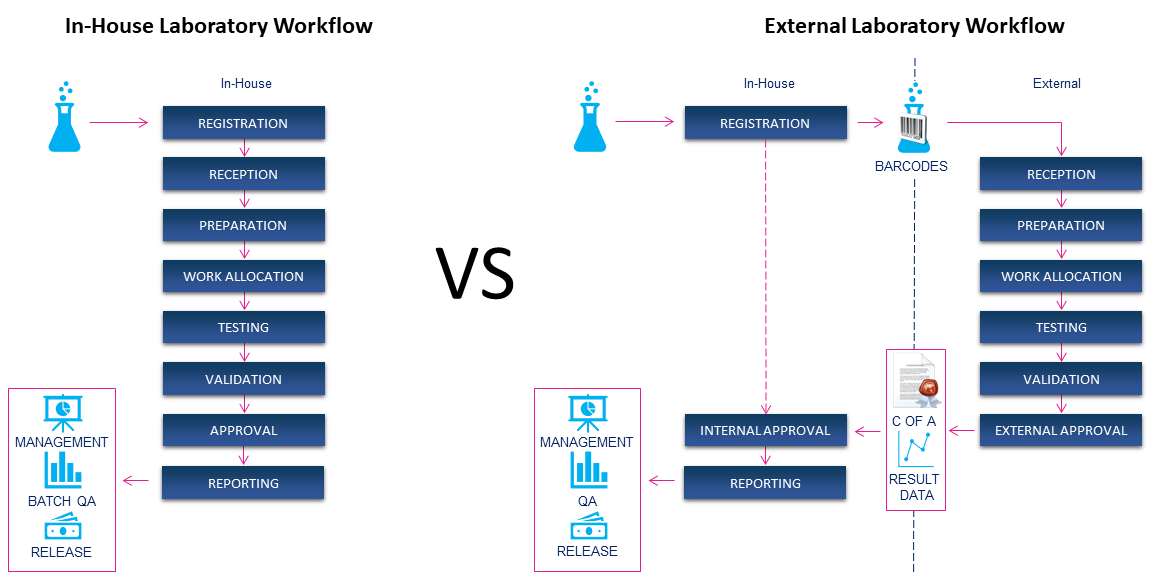
Determining the pros and cons of operating an in-house laboratory or sending samples out to an external laboratory to be tested is increasingly topical. Whether your products are toys or fish pies, cars or shoes, blood products or water, nearly all testing can be performed by a contract laboratory. But It is a mistake to think that outsourcing your laboratory means you do not need a system for tracking samples and the resulting data. [FIND OUT WHY]
11/04/2020 - The Secrets of Successful LIMS Configuration  ABOUT THIS WEBINAR: Are you interested in learning the secrets of LIMS configuration to allow easier modifications, extensions and upgrades? How can you ensure your implementation is successful? Learn what drives successful LIMS projects, unwrap the secrets of configuration, and discover the pitfalls to avoid. [LEARN MORE] ABOUT THIS WEBINAR: Are you interested in learning the secrets of LIMS configuration to allow easier modifications, extensions and upgrades? How can you ensure your implementation is successful? Learn what drives successful LIMS projects, unwrap the secrets of configuration, and discover the pitfalls to avoid. [LEARN MORE] | | 10/27/2020 - Interoperability challenges in the cybersecurity information sharing ecosystem  Cybersecurity and how it's handled by an organization has many facets, from examining existing systems, setting organizational goals, and investing in training, to probing for weaknesses, monitoring access, and containing breaches. An additional and vital element an organization must consider is how to conduct "effective and interoperable cybersecurity information sharing." As Rantos et al. note, while the adoption of standards, best practices, and policies prove useful to incorporating shared cybersecurity threat information, "a holistic approach to the interoperability problem" of sharing cyber threat information and intelligence (CTII) is required. The authors lay out their case for this approach in their 2020 paper published in Computers, concluding that their method effectively "addresses all those factors that can affect the exchange of cybersecurity information among stakeholders." Cybersecurity and how it's handled by an organization has many facets, from examining existing systems, setting organizational goals, and investing in training, to probing for weaknesses, monitoring access, and containing breaches. An additional and vital element an organization must consider is how to conduct "effective and interoperable cybersecurity information sharing." As Rantos et al. note, while the adoption of standards, best practices, and policies prove useful to incorporating shared cybersecurity threat information, "a holistic approach to the interoperability problem" of sharing cyber threat information and intelligence (CTII) is required. The authors lay out their case for this approach in their 2020 paper published in Computers, concluding that their method effectively "addresses all those factors that can affect the exchange of cybersecurity information among stakeholders." | | Building a Cybersecurity Toolkit  This is a University of Washington-created course that is released on the edX platform. The self-paced six-week course is designed to help learners to better understand the "type of characteristics and skills needed for cybersecurity jobs and to provide a realistic outlook on what they really need to add to their 'toolkits'—a set of skills that is constantly evolving, not all technical, but fundamentally rooted in problem-solving." The course is free to take, with a Verified Certificate of completion available for $199. The course requires on average two to five hours a week of effort. Access to the class began September 15, 2020. This is a University of Washington-created course that is released on the edX platform. The self-paced six-week course is designed to help learners to better understand the "type of characteristics and skills needed for cybersecurity jobs and to provide a realistic outlook on what they really need to add to their 'toolkits'—a set of skills that is constantly evolving, not all technical, but fundamentally rooted in problem-solving." The course is free to take, with a Verified Certificate of completion available for $199. The course requires on average two to five hours a week of effort. Access to the class began September 15, 2020. | | 11/19/2020 - Webinar - The Secrets of Successful LIMS Configuration
12/02/2020 - CSols Webinar: LabWare LIMS User Experience: 5 Quick Wins
12/16/2020 - Astrix Webinar – Leveraging Waters Empower 3 CDS Calculations to Assure Data Integrity in your Chromatography Lab | | 10/21/2020 - Regulatory compliance expectations for LIMS applications in the modern world
09/21/2020 - Staffing a new LIMS system effectively
09/14/2020 - Instrument Integration with LIMS
08/27/2020 - Best Software For Pathology Lab Management System
07/24/2020 - Electronic Lab Notebook | | 11/03/2020 - Sengenics Launches SEROMAX, an At-Home, Antibody Sample Collection Kit That Will Revolutionise Patient Recruitment and Monitoring in Clinical Trials
11/03/2020 - Dotmatics collaborates with LabVoice to enable voice-assisted laboratory workflows | | 11/12/2020 - Request for Proposal: Surveillance Testing for COVID
11/19/2020 - Request for Proposal: Electronic Health Record
11/30/2020 - Request for Proposal: Utah Department of Agricluture and Food for Laboratory Information Management System for their Lab
11/30/2020 - Request for Information: Centralized National Reporting Mechanism for COVID-19 Laboratory Tests Results and Other Reportable Conditions, United States
12/01/2020 - Request to Participate: Supply, Delivery, Implementation, Installation, Testing & Commissioning of NPHL (National Public Health Laboratory) LIS (Laboratory Information System)
12/07/2020 - Invitation to Tender: Toxicology Testing Equipment and LIMS Software for Drug Driving in the UK | | | E-mail your feedback or questions to us at: newsletter@limsforum.com Mail us: LabLynx, Inc. P.O. Box 673966 Marietta, GA 30006 Telephone: 866-LabLynx (522-5969) | |