Drug Companies Cited Over Lack of Stability Testing
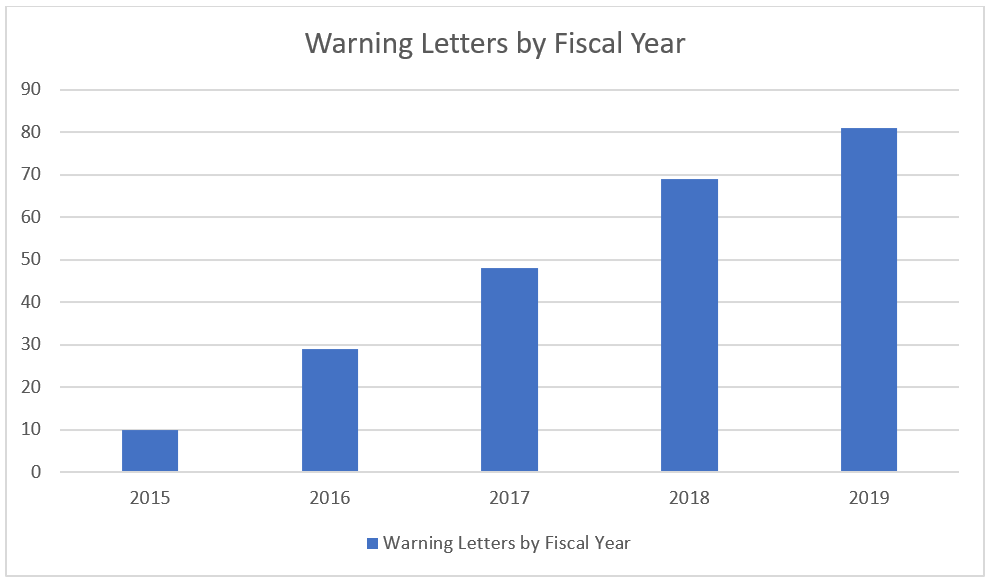 Warning letters and citations from the US FDA on the rise in the pharmaceutical industry. With approximately one in five warning letters in 2018 and 2019 mentioning 21 CFR Part 211 section 166 (Stability Testing) this part of the regulations also appears to be on the rise.
Warning letters and citations from the US FDA on the rise in the pharmaceutical industry. With approximately one in five warning letters in 2018 and 2019 mentioning 21 CFR Part 211 section 166 (Stability Testing) this part of the regulations also appears to be on the rise.
Stability testing has a key role in managing drug safety and efficacy. Learn how to create a defensible framework for stability testing in this article.
















