Stability Study Software Takes Centre Stage at Stability VI Symposia
Stability VI Symposia – London, UK, 30 June 2022
Matrix Stability, Autoscribe Informatics’ software solution for managing stability and shelf-life studies is to be demonstrated at the Stability VI Symposium hosted by the Royal Society of Chemistry (RSC) in London. Matrix Stability provides a complete stability management system. Users can create stability protocols that define all required stability study attributes including pull schedules, cycles, storage conditions and test requirements for specific batches. Stability studies can also be automatically linked to product batches that have been evaluated using Autoscribe’s Matrix Manufacturing Solution.
Stability VI is essential for anyone with an interest in the management and assessment of drug stability. The event is organised by the Joint Pharmaceutical Analysis Group (JPAG) and is jointly sponsored by the Royal Pharmaceutical Society and the Royal Society of Chemistry. Attendees will learn about the practical issues and challenges faced in managing stability work, and the basics of method development and testing.
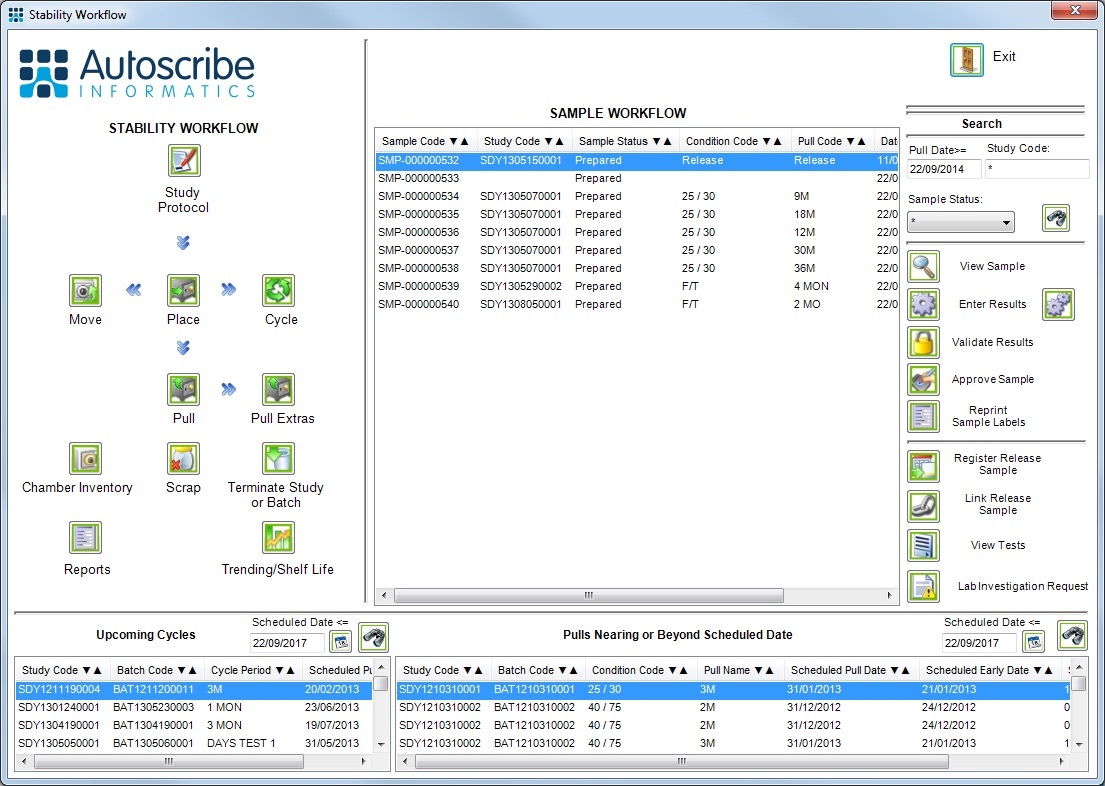
A set of standard reports delivered with the system can create the necessary documentation for the stability protocol and its associated results. The system can, optionally, generate projected shelf-life trend analysis from the stored test results. The statistics and charting capabilities include ANCOVA statistics, tests for poolability, out of trend detection and residuals analysis. Predictions can be made using pooled, pooled slope, and worst-case data.
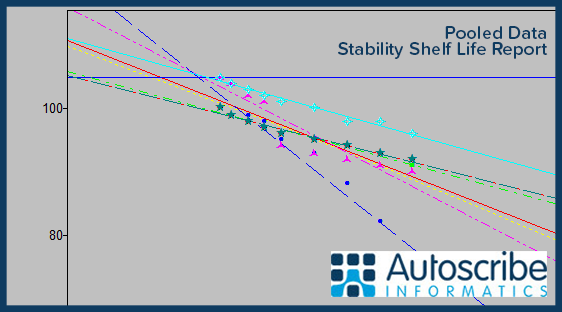
Simon Wood, Product Manager at Autoscribe Informatics, said: “Matrix Stability provides a solid foundation for drug stability studies in the pharmaceutical industry. The scheduling functions vastly simplify and automate the management of stability programmes and ensure critical pull points are never missed. Creating industry standard shelf-life statistics from the test results recorded within the built in LIMS functionality allows entire study programmes to be managed by a single system. Matrix Stability manages the full lifecycle of stability studies and provides the support required to meet regulatory requirements including GMP and the FDA 21 CFR Part 11.”
Further information about the Stability VI Symposia can be found here:
https://www.jpag.org/?p=meetings&r=155
Further information on Matrix Stability is available from the website:
https://www.autoscribeinformatics.com/lims/stability
















