What Is IQ OQ PQ in Software Validation
 If you work in a business regulated by a government agency, once you’ve implemented or upgraded your LIMS, ELN, or CDS, you need to validate it for your intended use. One part of the validation process that often seems confusing to our clients is the IQ, OQ, PQ testing. This blog will demystify these validation protocol test scripts.
If you work in a business regulated by a government agency, once you’ve implemented or upgraded your LIMS, ELN, or CDS, you need to validate it for your intended use. One part of the validation process that often seems confusing to our clients is the IQ, OQ, PQ testing. This blog will demystify these validation protocol test scripts.
When your laboratory operates in one of the regulated businesses, such as pharmaceuticals or food and beverage, it is critical that you validate your lab informatics system to ensure that it is fit for purpose. When a regulatory agency (such as the U.S. Food and Drug Administration or Health Canada) comes to audit your facility, you will need to have the proper documentation that shows you have performed this validation. There is a lot of documentation needed to complete the picture of a validated system; however, when it comes to providing the evidence that your system operates as intended and is fit for purpose, validation protocol test scripts are critical.
What Are Validation Protocol Test Scripts?
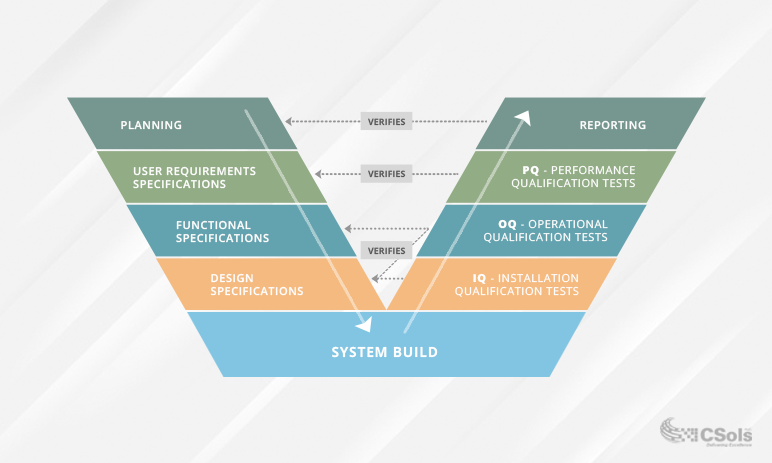
Validation protocol test scripts provide a standard framework for the testing process. Each step in the testing process verifies specific pieces of the planning and specifications that were used to design the system. This is seen in the classic V model that is used in the GAMP guidelines. The left side of the model addresses the requirements and specifications that are required to define and build a system. The right side of the model addresses the associated testing that is required to verify the requirements and specifications.
WHAT ARE IQ (INSTALLATION QUALIFICATION) TEST SCRIPTS?
IQ test scripts ensure that the laboratory informatics system is received and properly installed in your environment based on the vendor installation requirements, and that the environment is suitable for the operation of the laboratory informatics system. The system is usually first set up in a test or validation environment to perform this testing. Vendors often provide IQ test scripts and associated documentation; however, that documentation should be reviewed before it’s executed to make sure there are no changes required to address your specific environment.
WHAT ARE OQ (OPERATION QUALIFICATION) TEST SCRIPTS?
OQ test scripts demonstrate that the laboratory informatics system will function according to the operational specifications in its final operating environment, based on how it was developed. This testing confirms that all functionality defined in the Functional Requirements Specification is present and working as it should, and that there are no bugs. OQ testing also confirms that design elements not tested during IQ testing, such as configuration and customization, also work as they should. Vendors often provide OQ test scripts and associated documentation for the out-of-the-box functionality of their system, based on how they developed it. However, once installed, if there are any configurations and/or customizations made for your specific intended use, that functionality should also be addressed in supplemental OQ test scripts to verify it meets the functional specifications.
WHAT ARE PQ (PERFORMANCE QUALIFICATION) TEST SCRIPTS?
PQ test scripts (sometimes referred to as user acceptance testing) demonstrate the overall intended use of the system according to your procedures and processes. They are derived from the User Requirements Specification and should reflect the day-to-day or end-to-end use of the system in your environment. PQ test scripts are executed in an environment that simulates your production environment. PQ testing also verifies that the LIMS, ELN, or CDS is fit for its intended purpose, operates in accordance with defined standard operating procedures, performs according to the user requirements, and complies with 21 CFR Part 11. This testing will also look at the support environment within which the system is to operate, for example training or maintenance. Vendors do not often provide PQ test scripts or associated documentation, as it is based on your intended use, processes, and procedures.

When And How Should You Perform IQ OQ PQ Testing?
All testing is done after the system requirements have been gathered and the system is ready to be installed. A test plan or validation plan should include the scope of the testing and the testing levels, which are determined by a risk assessment. The test plan also includes staffing and software requirements, roles and responsibilities, and reports and metrics.
The testing itself consists of test cases that are written specifically to address each aspect of the user and functional requirements. The order of testing starts with the IQ, then the OQ, and finally the PQ. It is important that the installation of the system be verified before any functional testing is performed. It is a best practice that any performance testing occurs after operational testing so that any potential issues with basic functionality can be addressed before verifying end-to-end testing of your processes.

Why Do I Need IQ OQ PQ?
Proper IQ, OQ, and PQ testing is critical to demonstrate to any regulatory body that your LIMS, ELN, or CDS has been properly validated and that it is fit for your intended purpose. This is necessary if your company desires to do business in one of the regulated industries. Failure to properly validate your laboratory informatics system can result in unnecessary rework at best and in an FDA audit or production shutdown at worst.
Understanding the role of IQ, OQ, and PQ testing and how each relates to specific parts of the validation process can help you get the most out of your laboratory informatics investment.
Do you have more specific questions about IQ, OQ, PQ test scripts and how they should be applied in your validation process? If so, share them below.
https://www.csolsinc.com/blog/what-is-iq-oq-pq-in-software-validation/
















