An assessment of solvent residue contaminants related to cannabis-based products in the South African market
| Full article title | An assessment of solvent residue contaminants related to cannabis-based products in the South African market |
|---|---|
| Journal | Journal of Cannabis Research |
| Author(s) | Viviers, Hendrik J.; Petzer, Anél; Gordon, Richard |
| Author affiliation(s) | National Analytical Forensic Services, North West University |
| Primary contact | Email: henrick at nafs dot co dot za |
| Year published | 2022 |
| Volume and issue | 4 |
| Article # | 19 |
| DOI | 10.1186/s42238-022-00130-3 |
| ISSN | 2522-5782 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://jcannabisresearch.biomedcentral.com/articles/10.1186/s42238-022-00130-3 |
| Download | https://jcannabisresearch.biomedcentral.com/track/pdf/10.1186/s42238-022-00130-3.pdf (PDF) |
Abstract
Organic solvents are used for manufacturing herbal medicines and can be detected as residues of such processing in the final products. It is important manufacturers control the presence of these solvent residues for the safety of consumers. South African cannabis-based product samples were analyzed for solvent residue contaminants as classified by the United States Pharmacopeia (USP), chapter 467. The origin of these samples ranged anywhere from crude extract to product development samples and market-ready final products. Samples were submitted to a contract laboratory over a period of two years, from 2019 to 2021. To date, no data of this kind exist in South Africa, specifically relating to cannabis-based medicinal, recreational, or complementary products.
A total of 279 samples was analyzed in duplicate by full evaporation headspace gas chromatography–mass spectrometry (GC–MS), and the results were reported in an anonymized format. The results showed an alarming 37% sample solvent residue failure rate with respect to adherence to the USP 467 specification. This research highlights the importance of ensuring regulations are enforced to control product quality. Additionally, it highlights how the South African public need to be educated about the risks associated with cannabis-based products.
Keywords: residual solvents, ethanol, isopropanol, South Africa, medicinal cannabis, chromatography, GC–MS, cannabis oil, plant extracts
Background
It is imperative to subject herbal preparations, medicines, and recreational products to quality control. For the pharmaceutical industry—as well as cannabis in general—there are an abundance of control measures in place to ensure product safety and efficacy.[1][2] A range of organic solvents are used for manufacturing herbal medicines and can be detected as residues of such processing in the final products. Medicinal cannabis extracts and other processed forms may thus contain residual solvents. This is especially relevant to extracts, which have a sticky and viscous nature that make it difficult to remove solvents.[3] The most common examples of such cannabis extracts are termed “Rick Simpson oils” or “FECOs” (full extract cannabis oils).
Cannabinoids, as well as terpenoids and flavonoids, are extracted by a solvent, followed by an evaporation step in order to increase the concentration of these compounds in the extract.[3][4] These types of cannabis oils or extracts are becoming increasingly popular amongst self-medicating patients because of the simplicity and low cost involved in producing the oils.[3] After solvent evaporation, residues are still present in the extract, and the concentrations of the solvent residues should be controlled through good manufacturing practice (GMP) and quality control of the final products.[5] To ascertain whether a product is safe for chronic human consumption, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), as well as the United States Pharmacopeia (USP), have listed predetermined solvent residue limits. Solvents are classified into the following categories by the ICH[5] and the USP[6] according to their potential risks:
- Class 1: Solvents to be avoided as they are potentially carcinogenic, such as benzene;
- Class 2: Solvents with toxic potential, such as methanol or acetonitrile; and
- Class 3: Solvents with limited toxic potential, such as ethanol.
Residual solvents are primarily analyzed by headspace gas chromatography–flame ionization detection (GC-FID) or liquid injection GC-FID.[6] The alternative use of mass spectrometry (MS) detection may provide additional selectivity for co-eluting solvents.
In this study, a published analytical method was employed as basis for the development of the final analysis method for residual solvents.[7] As a result of the viscosity of most of the cannabis extracts, liquid injection is not feasible. Although the ICH and USP provide a guideline that lists the solvents which should be included in a working list[5][6], the solvents that inevitably get analyzed are ultimately decided by each manufacturer or quality control laboratory.
Based on interaction with cannabis extract manufacturers, Table 1 was compiled and provides a list of the solvents that have been analyzed for each class. This list is by no means exhaustive and could be altered to include additional solvents. Few manufacturers employ Class 1 and 2 solvents; nonetheless, they are included here.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
An evaluation of the quality of medicinal cannabis-based products has been performed previously[4],, but it was only limited to samples in the Netherlands. This study included solvent residue testing but only to the extent of comparing different production methods with one another using different types of solvent. It should be noted that due to the high viscosity of the extracts, significant solvent residues were also reported by this study.[3] A study evaluating the quality of artisanal/home produced cannabis-based products, used to manage seizures in children, was conducted in 2022.[8] Of the 58 cannabis samples evaluated, 17 (29%) contained concentrations of ethanol and isopropanol above USP solvent residue limits.[8] A study conducted in 2015 found that isopentane was the most frequently detected (29.8%) solvent residue in 57 samples.[9] Other residual solvents detected were butane, heptane, hexane, isobutane, isopropanol, neopentane, pentane, and propane.[9] It should be noted that this article only reported solvent residues detected and not solvent residues above a specified limit.
With this background, the aim of this study was to analyze a segment of the South African cannabis-based products in circulation and to provide a detailed overview of the solvent residues contained in these products. Furthermore, the adherence of these samples to the imposed limits by the ICH and USP will also be evaluated.[5][6] To date, no data of this kind exists in South Africa. The output of the study will provide insights to manufacturers, the public, and regulators alike.
Materials and methods
An assortment of samples from a variety of manufacturers in South Africa was submitted to a contract laboratory for analysis. Manufacturers are defined as any type of user, retailer, reseller, producer, or importer of cannabis-based products. Whether these manufacturers maintain the full value chain or only a portion thereof, they are defined as manufacturers for the purpose of this study. Manufacturers may include cultivators of plants, producers of products, importers, resellers, and pharmaceutical manufacturers. The data for all those submitted samples are represented in an anonymized format. Solvents from Classes 1, 2, and 3 were analyzed (see Table 1). Depending on the solvents employed in the manufacturing process, the appropriate solvent class was chosen by each manufacturer for each specific sample submission. A screen of all available solvents would be impractical; thus, manufacturers selected the solvent class which was most likely present in the final product. In total, 299 samples were analyzed in duplicate, for a total of 598 data points. Consent was provided to employ the data for research purposes. The majority of samples submitted for residual solvent analysis could be defined as any sample prepared or processed in a way to extract cannabinoids from plant material to up-concentrate the cannabinoid content in the final product. These might include full extract cannabis oil (FECO), Rosin, Rick Simpson oil (RSO), Hashish, Butane hash oil (BHO), etc.
A Clarus 680 gas chromatograph equipped with a SQ8 mass spectrometer and HS40 Headspace autosampler was obtained from Perkin Elmer Corporation (Waltham, MA, USA) and employed for headspace gas chromatography–mass spectrometry (HS-GC-MS) analysis. A Zebron ZB-624 (30 m, 0.32 mm × 1.8 μm) capillary column obtained from Phenomenex (Torrance, CA, USA) was employed for separation. Two reference methods were employed as a starting point and were altered to achieve more desirable run times.[6][7] Sample preparation was conducted as described by Hilliard et al.[7] Residual solvent certified reference materials (CRMs) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
The following headspace instrumental parameters were employed: needle temperature 175 °C, vail oven temperature 170 °C, transfer line temperature 175 °C, GC cycle time 32 minutes, injection volume 20 μL, pressurize time 1.0 minute, thermostat time 20 minutes, withdraw time 0.1 minutes. GC parameters included: injection port temperature 220 °C, split ratio 5:1, carrier gas: (helium) flow 1.2 mL/min. The GC oven program initial temperature was set to 44 °C hold for 16 minutes, ramp 1–12 °C/minute to 153 °C, ramp 2–50 °C /minute to 230 °C hold for 1.5 minutes. The MS transfer line was set to 200 °C with the source set at 150 °C. Table 2 shows the MS m/z ions monitored.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
A limit test procedure was employed for the analysis of all residual solvents. A calibration standard was prepared at the concentration limit of each individual solvent using the first batch of CRM. A one-point calibration was employed for each USP limit of each individual solvent. A second batch of CRMs was employed to prepare a control standard, again at the limit concentration stipulated by the USP 467 monograph.[6] Analysis commenced with a blank run, then a calibration standard, followed by a control standard every 10 injections, to avoid instrumental drift. Sample sets were concluded by a control standard to ensure all samples within a sample set adhered to bias and variation limits. Control standard specifications were 15% RSD at the concentration limit, and re-calibration was performed when control standards fell outside these specifications. This method was regarded as quantitative with a very narrow range of 15% across the USP 467 concentration limit. Any values falling outside this window were regarded as semi-quantitative only. Since manufacturers are only interested in knowing whether the products fall within a certain specified concentration limit, a full quantitative analysis was redundant.
The data was subdivided into three different categories:
- 1. Individual solvent analytes were grouped together with no relationship to the sample. This was done to determine the number of times this specific solvent failed to adhere to the USP and ICH specification.[5][6] Furthermore, the number of times a certain solvent was present, irrespective of adherence to a specification limit, was also determined and noted as either present when it occurred or not detected (ND).
- 2. Secondly, samples were grouped together to determine the frequency of sample failures as well as the frequency by which the test panel solvents are present in a specific sample. The frequency of failed samples only considered whether any one of the solvents in the test panel failed.
- 3. Lastly, with the samples grouped together, the total amount of solvent detected in the sample was summed together and compared to a set adherence limit. As such, even if individual solvents within a sample adhered to the USP specification limit, the sum total concentration of solvent present in a sample may exceed the set adherence limit.
Results
The results of the analyses are shown in the Appendix (Table 3). The results are grouped according to individual solvent analytes and shows the occurrence of each individual solvent across the 298 samples. The Appendix (Table 4) also shows sample failures as well as whether a solvent was present in the sample irrespective of the safety limit. Finally, the Appendix (Table 5) shows sample failures when summed solvents concentrations are considered against a safety limit.
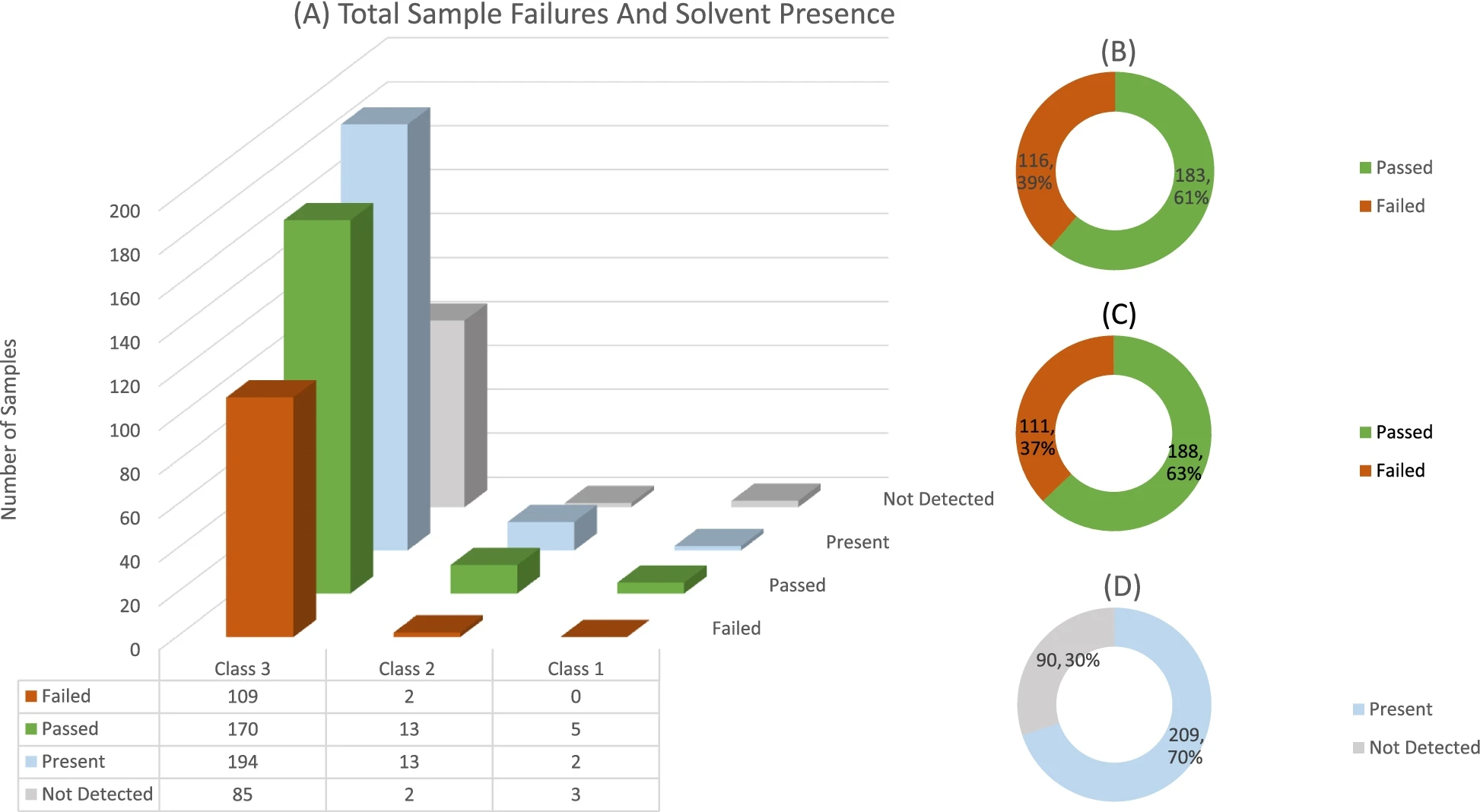
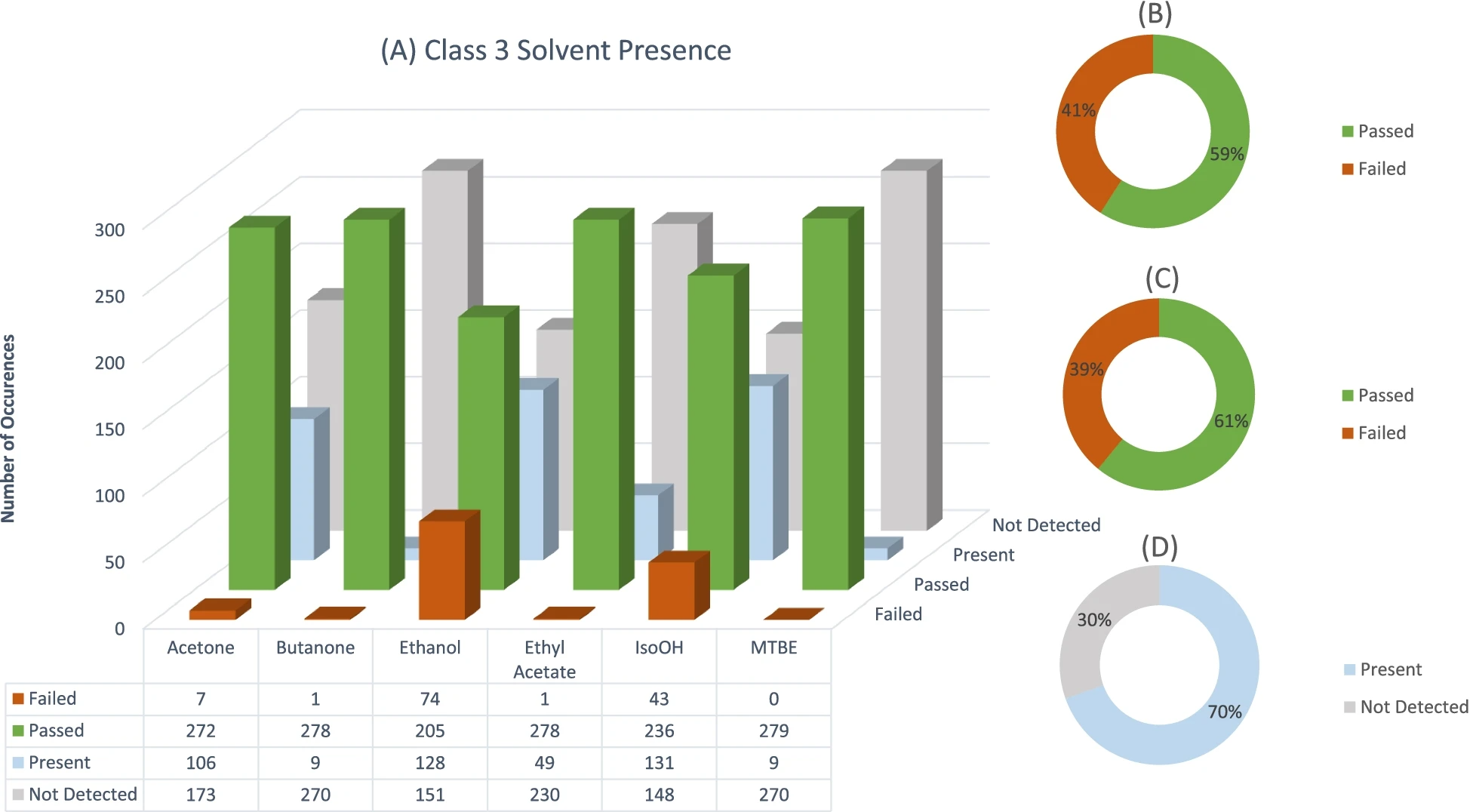
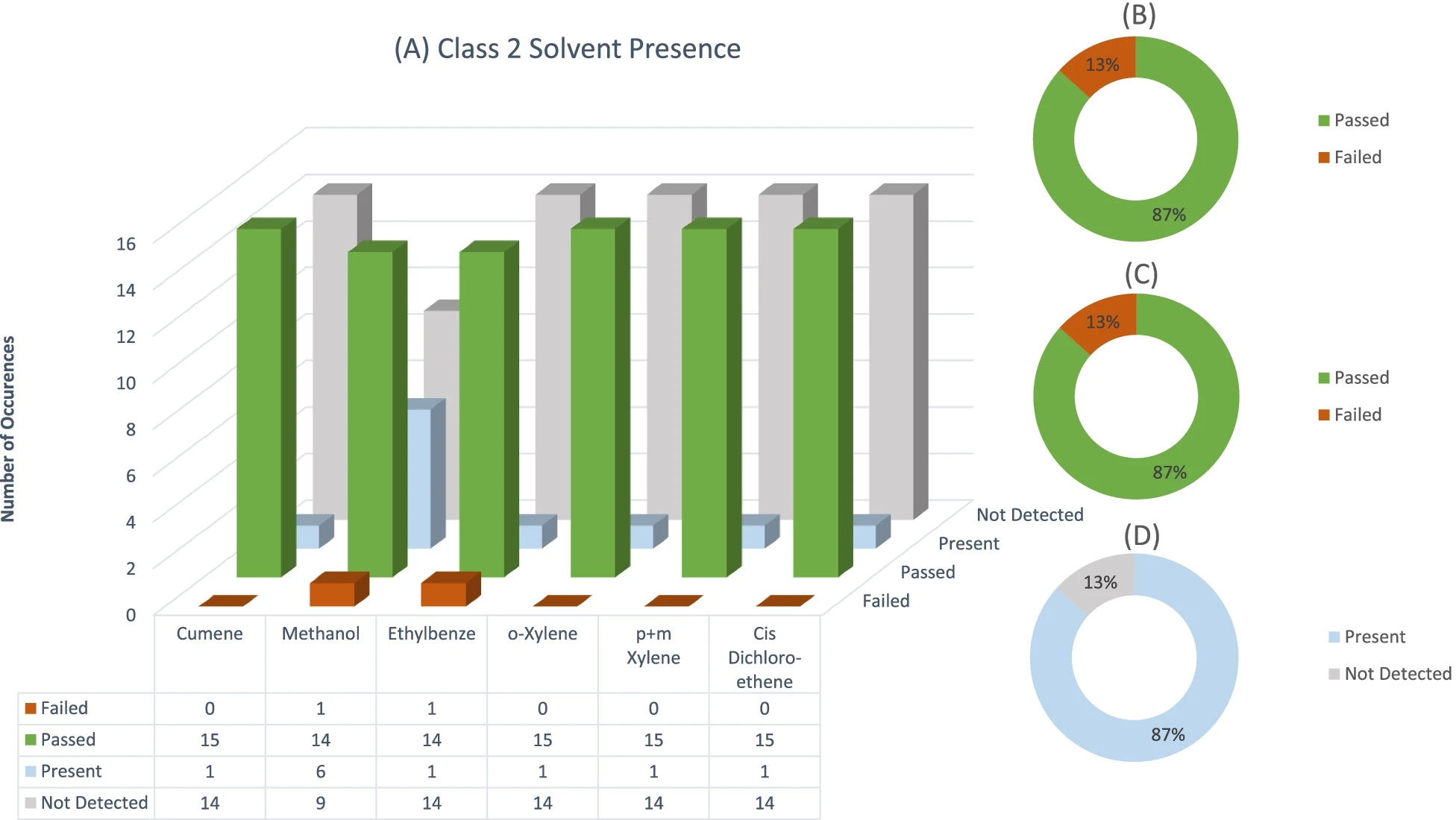
Figure 1 shows the individual solvent failures, as well the number of times each solvent occurred or was detected for all three solvent classes. Since most of the samples that were analyzed contain Class 3 solvents, Figs. 2 and 3 illustrate the number of failures as well as the occurrence of each individual solvent in Class 2 and Class 3. It should be noted that for Figs. 2 and 3, only solvents which were present are displayed. Additionally, pie graphs B, C, and D found in all three figures show the amount of sample failures, the amount of sample failures if concentrations are summed, and finally the overall occurrence/detected solvents in all samples, respectively.
|
|
|
Discussion
The data presented herein represents an overview of cannabis-based products in South Africa that were screened for solvent residues. This data should not be regarded as a representation of the entire South African cannabis market since not every product on the market was analyzed. Each solvent class will be discussed individually starting with the solvents that were present in most samples. Solvents that were not detected in any of the samples but were included in the test panel are omitted from the figures.
Class 3
Class 3 solvents occurred in most of the samples. Solvent residues in this class have the least stringent specification limits.[5][6] This can be viewed in a positive light since most samples analyzed fell within this class and can be considered the least dangerous. When considering individual solvent analytes, the most prevalent solvent residue failure was ethanol, followed by isopropanol and acetone (see Fig. 2). When considering the presence of these residues in samples, ethyl acetate should also be mentioned. Although ethyl acetate had only one residue failure, it was present in almost 50 samples. Additionally, although isopropanol had fewer failures than ethanol, isopropanol residues were present in more samples than ethanol. A reason for the prevalence of these solvent residues might be the ease of acquiring such solvents from a variety of vendors in South Africa. A variety of manufacturers also do not employ food-grade or high-purity ethanol, which contains denaturants like ethyl acetate and acetone, since high-purity ethanol is taxed at a significant rate in South Africa. Comparing individual Class 3 solvent residue failures to summed solvent residue failures, summed solvent residue failures only increased by a small fraction (39% failed individual vs. 41% failed summed). It could be argued then that most manufacturers employ a single moderately pure solvent in their production process. A high percentage of Class 3 samples (69.5%) contained detectable concentrations of solvent residues even though these residues were not above the specification limit.
Class 2
Class 2 solvents were second most prevalent in the sample dataset. Solvent residues in this class have more stringent specification limits.[5][6] These solvents should be viewed with concern by home producers as they are more dangerous to human health. Considering the individual Class 2 solvents, only one failure occurred for each of methanol and ethylbenzene (see Fig. 3), yielding a 15% failure rate. A high percentage of Class 2 samples (87%) contained detectable concentrations of solvent residues even though these residues were not above the specification limit. Although these solvents pose higher health risks, the lower failure rate of Class 2 solvents is a positive finding since fewer manufacturers employ these solvents. What should be noted is the much higher percentage of Class 2 solvents that are present in final products submitted for this test panel. This might translate to Class 3 solvents being easier to remove during manufacturing than Class 2 solvents. Alternatively, because of the lower detection limits needed to detect Class 2 solvents compared to Class 3 solvents, they are detected at a higher frequency in samples.
Class 1
Class 1 solvents occurred the least in the samples. Solvent residues in this class have the most stringent specification limits, and should be avoided.[5][6] These solvents are also recognized as carcinogens.[10] The samples were only screened for the presence of benzene in this category and a 0% failure rate occurred, even with an imposed specification limit of 2 ppm ( see Table 3 and Table 4 in the Appendix). Benzene was present in only two samples, and it should be noted that in both instances benzene concentrations were below the specified 2 ppm USP/ICH limit.[5][6]
Concerning all classes
The data of all solvent classes were pooled as shown in Fig. 3, which indicates the total number of samples submitted for any class solvent residue analysis that failed. Among the 279 samples, a total of 111 samples had at least one solvent analyte that failed the USP and ICH specification limit (37% failure rate). Note that a study conducted on solvent contaminants in cannabis-based products employed to treat epilepsy averaged a USP specification failure rate of 29%[8], compared to the significantly higher South African failure rate of 37%.
For summed solvent concentrations, the failure rate increased by a marginal 2%, which could be interpreted to mean that manufacturers use a single moderately pure solvent during their production. This is supported by the finding that only high-failing concentrations of a single solvent are usually present in a specific sample. The overall presence of any solvent in a sample is displayed in Fig. 3. A high percentage of samples (70%) contained detectable concentrations of solvent residues even though these residues might not have been above the specification limit.
Another study conducted in 2015 found solvent detection percentages of 71.9%[9], comparable to the South African 70%. It should also be noted that not all samples submitted employed solvent extraction as means of manufacturing. A limited number of samples were submitted to obtain a certificate of analysis, which shows compliance to USP 467 or ICH Q3C, even though no solvent was used in production.
Conclusion
In conclusion, when assessing solvent residues present in samples against a set of pharmacopeial safety limits, it is evident that a large fraction of cannabis-based products in South Africa exceeds these limits. These findings are either comparable or show higher failure rates than other studies published.[8][9] Even though safety limits for solvent residues have been published, adherence by manufacturers is lacking, notwithstanding enforcement of these limits by regulators. With a 37% overall sample solvent residue failure rate, it is alarming that these products are being distributed in South Africa. It is the aim with the publication of this data to inform the public and regulators alike concerning these issues.
Abbreviations, acronyms, and initialisms
BHO: butane hash oil
CRM: certified reference material
FECO: full extract cannabis oil
GC-FID: gas chromatography-flame ionization detection
GMP: good manufacturing practice
ICH: International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
MS: mass spectrometry
ND: not detected
RSD: relative standard deviation
RSO: Rick Simpson oil
USP: United States Pharmacopeia
Appendix
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Acknowledgements
National Analytical Forensic Services (NAFS) Laboratory. Laboratory staff: Paul Myburgh, Jeanette Leygonie, Alexander Wrbka, Gerdus Kemp (CEO).
Author contributions
HJ Viviers (corresponding author): conceptualization, methodology, investigation, data curation, writing—original draft preparation. A. Petzer: supervision, writing—reviewing, and editing. R. Gordon: supervision, writing—reviewing, and editing. All authors read and approved the final manuscript.
Funding
No funding was received for this study.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to the data being anonymized contract laboratory samples. The summarized data is available from the corresponding author at henrick at nafs dot co dot za on reasonable request. It should be noted however that either certain sample details will be redacted, or not be available. Raw data reports are not available.
Competing interests
Author H.J. Viviers is employed at the National Analytical Forensic Services (NAFS), and the authors declare the financial interests/personal relationships may be considered as potential competing interests. NAFS did not play any role in the design and conduct of the study, writing of the manuscript, or decision to publish. The data was accumulated by the analysis of routine samples submitted to the laboratory. Consent was provided by the laboratory and clients to use and publish data in anonymized format.
References
- ↑ World Health Organization, ed. (2007). WHO guidelines for assessing quality of herbal medicines with reference to contaminants and residues. Geneva: World Health Organization. ISBN 978-92-4-159444-8. OCLC 232540335. https://www.worldcat.org/title/mediawiki/oclc/232540335.
- ↑ Viviers, Hendrik Jacobus; Petzer, Anél; Gordon, Richard (1 May 2021). "An assessment of the potency related to Cannabis-based products in the South African market" (in en). Forensic Science International 322: 110754. doi:10.1016/j.forsciint.2021.110754. https://linkinghub.elsevier.com/retrieve/pii/S0379073821000748.
- ↑ 3.0 3.1 3.2 3.3 Romano, L.L.; Hazekamp, A. (2013). "Cannabis Oil: chemical evaluation of an upcoming cannabis-based medicine". Cannabinoids 1 (1): 1–11. http://www.cannabis-med.org/index.php?tpl=cannabinoids&id=276&lng=en&red=cannabinoidslist.
- ↑ 4.0 4.1 Hazekamp, A. (2006). "An evaluation of the quality of medicinal grade cannabis in the Netherlands". Cannabonoids 1 (1): 1–9. http://www.cannabis-med.org/index.php?tpl=cannabinoids&id=144&lng=en&red=cannabinoidslist.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 5.9 European Medicines Agency (9 August 2019). "ICH guideline Q3C (R6) on impurities: guideline for residual solvents" (PDF). European Medicines Agency. https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-33.pdf.
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 The United States Pharmacopeial Convention. "<467> Residual Solvents". United States Pharmacopoeia. The United States Pharmacopeial Convention. https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/revisions/gc-467-residual-solvents-ira-20190927.pdf.
- ↑ 7.0 7.1 7.2 Hilliard, C.; Rigdon, A.; Schroeder, W. et al. (July 2014). "A Fast, Simple FET Headspace GC-FID Technique for Determining Residual Solvents in Cannabis Concentrates" (PDF). Restek Corporation. https://pdf2.chromtech.net.au/A%20Fast,%20Simple%20FET%20Headspace%20GC-FID_FFAN2009A-UNV.pdf.
- ↑ 8.0 8.1 8.2 8.3 Suraev, Anastasia; Benson, Melissa J.; Martin, Lewis; Lintzeris, Nicholas; McGregor, Iain S. (1 February 2022). "Determination of contaminants in artisanal cannabis products used for childhood epilepsy in the Australian community: A sub-analysis of the ‘PELICAN’ study" (in en). Epilepsy & Behavior 127: 108496. doi:10.1016/j.yebeh.2021.108496. https://linkinghub.elsevier.com/retrieve/pii/S1525505021007575.
- ↑ 9.0 9.1 9.2 9.3 Raber, Jeffrey C.; Elzinga, Sytze; Kaplan, Charles (2015). "Understanding dabs: contamination concerns of cannabis concentrates and cannabinoid transfer during the act of dabbing" (in en). The Journal of Toxicological Sciences 40 (6): 797–803. doi:10.2131/jts.40.797. ISSN 0388-1350. https://www.jstage.jst.go.jp/article/jts/40/6/40_797/_article.
- ↑ Baker, Edward L. (1 October 1994). "A Review of Recent Research on Health Effects of Human Occupational Exposure to Organic Solvents: A Critical Review" (in en). Journal of Occupational and Environmental Medicine 36 (10): 1079–1092. doi:10.1097/00043764-199410000-00010. ISSN 1076-2752. http://journals.lww.com/00043764-199410000-00010.
Notes
This presentation is faithful to the original, with minor changes to presentation; grammar and spelling required more cleanup for improved readability. In some cases important information was missing from the references, and that information was added. The original article listed references in alphabetical order; this version lists them in order of appearance, by design.