Laboratory information management system for the biosafety laboratory: Safety and efficiency
| Full article title | Laboratory information management system for the biosafety laboratory: Safety and efficiency |
|---|---|
| Journal | Journal of Biosafety and Biosecurity |
| Author(s) | Sun, Dingzhong; Wu, Linhuan; Fan, Guomei |
| Author affiliation(s) | Institute of Microbiology Chinese Academy of Sciences |
| Primary contact | Email: wulh at im dot ac dot cn |
| Year published | 2021 |
| Volume and issue | 3(1) |
| Page(s) | 28–34 |
| DOI | 10.1016/j.jobb.2021.03.001 |
| ISSN | 2588-9338 |
| Distribution license | Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International |
| Website | https://www.sciencedirect.com/science/article/pii/S2588933821000042 |
| Download | https://www.sciencedirect.com/science/article/pii/S2588933821000042/pdfft (PDF) |
Abstract
The laboratory information management system (LIMS) has been widely used to facilitate laboratory activities. However, the current LIMS does not contain functions to improve the safety of laboratory work, which is a major concern of biosafety laboratories (BSLs). With significant biosafety information needing to be managed and an increasing number of biosafety-related research projects underway, it is worthy of expanding the current framework of LIMS and building a system that is more suitable for BSL usage. Such a system should carefully trade off between the safety and efficiency of regular lab activities, allowing laboratory staff to conduct their research as freely as possible while also ensuring their and the environment’s safety. In order to achieve this goal, relevant information on the type of research, laboratory personnel, experimental materials, and experimental equipment must be collected and fully utilized by a centralized system and its databases.
Keywords: laboratory information management system, biosafety, biological research laboratory, laboratory safety, workflow management
Introduction
A laboratory information management system (LIMS) is a computerized system that collects, processes, and stores laboratory-generated data and information. Though the LIMS was initially developed to automate the management of experimental data, it now has the potential to develop into the digital hub of many laboratory activities.[1][2][3][4] Inside the lab, by deeply entwining the LIMS and highly-automated laboratory equipment, researchers are even able to program robots (for example, the Biomek series from Beckman Coulter) to execute iterative experiments without any human operation.[5] Outside the lab, many current LIMS in clinical and analytical labs have greatly accelerated the process of releasing test results to the customer because of their network-based reporting system.[4] Regardless of the specific functions a LIMS possesses, the ultimate purpose of the LIMS is to save human labor and improve data quality (i.e., accuracy, reliability, and timeliness).[6]
Although the importance of a LIMS in a lab was quite low initially, with the rapid development of computer and network technologies, the LIMS had gradually evolved to play more diverse roles and become an irreplaceable part in many labs. The range of LIMS users has expanded as well, from mostly analytical labs to diagnostic biosafety laboratories (BSLs) of specific function, as well as more general research labs.[2][3][7][8]
Nevertheless, the functions of all current LIMS, to our knowledge, do not address the major concern of biological safety and security in biosafety labs. This is even true for those LIMS currently used in BSLs. Although the modern LIMS has reduced a number of human factors from certain experimental activities, few if any have actually increased the general safety of the BSL as these systems do not contain any safety- and security-control mechanisms. The focus of a traditional LIMS, whether it is used in a BSL or not, is still to better facilitate lab staff performing experiments. Even the electronic laboratory notebook (ELN), a tool widely appreciated by BSL staff, was not designed for but merely happened to suit the working environment of BSLs. As such, we do not currently have any digital systems that can help a BSL manage its biosafety information. At the same time, biosafety-related information has been collected, stored, and transmitted in digital form in BSLs since at least 2000, and the amount of digital biosafety information is growing, when compared to paper-based information.[9] This has resulted in a strange situation, one where biosafety information itself is highly digitalized, yet there is no centralized system designed to better organize that electronic information and enable laboratorians to use it appropriately. Most information typically stays in the form of isolated electronic documents on either a local computer or hosted on a local network, and the search and retrieval of the information is usually complicated. For example, when a safety document is requested by a user, the search, deployment, use, and dissemination of the document is largely done on an individual basis, via a not-well-indexed information system and an unrelated transmission system like email or even paper-based messaging. Hence, the information management system of biosafety labs—if the system even exists—is ineffective, inefficient, and cannot satisfy the needs of the modern biosafety lab. We therefore believe that a specific information management system for biosafety laboratories that is able to improve the safety and efficiency of these laboratories is a necessity.
A laboratory information management system for the biosafety laboratory
This paper proposes a laboratory information management system for the modern biosafety laboratory (BSL-LIMS), specifically designed for both high-level biosafety labs (BSL-3 or -4) and basic research BSLs. Since the BSL-LIMS has its root in the specific needs of BSLs, the focus of the system is different from the traditional LIMS. Biosafety and biosecurity are made the primary concerns in the BSL-LIMS, with similar if not more importance than improving efficiency.[10]
Biosafety in laboratories has two major considerations: the safety of those who work in the lab in some capacity and the safety of the overall environment.[11] As such, the aim of a BSL is to keep both the personnel and environment safe before, during, and after experiments and other analyses. If there are any unavoidable safety risks in the course of a research project, then the risks must be reduced to an acceptable and controllable degree before the start of the project, and be kept this way until the end of it; otherwise, the project should be abandoned rather than allowed to begin. Hence, as a part of BSL activities, the design of the BSL-LIMS has to comply with the safety standards set by BSLs. Since biosafety is a precondition for experiments in BSLs, the focus of the corresponding LIMS shifts from post-experimental information (i.e., results) management to pre-experimental information (i.e., preparation) management. Even though the overall framework of the BSL-LIMS might look similar to that of a traditional LIMS, their contents would be very different.
Information management in a BSL can be divided into four categories:
- Project management
- Personnel administration
- Experimental material management
- Equipment management
As such, any information management system would involve a large amount of information collection and exchange within and between different categories. Many checkpoints would need to be set up for the sake of safety control. These interconnected information flows and checkpoints would then form an elaborate network whose structure can be simplified to Petri Nets[2], with laboratory data or information as places and research or audit activities as transitions. Since laboratory workflow management has already been a part of many regular research labs’ information management systems[2][3][7], some modules in these traditional LIMS (e.g., the management of samples, materials, or equipment) may be used by the BSL-LIMS, though new databases on biosafety information should be added, and existing structures probably need to be redesigned in order to meet the new demands for improving biosafety and biosecurity.
Project management
Research projects comprise the bulk of a research laboratory's activities. In other words, a research lab is founded so as to support a succession of research project. Hence, the effective management of projects is the most important job for a lab manager or director. However, there are two new challenges to meet to get the job done in high-level BSLs, in addition to the challenges raised in regular laboratories.
First, high-level BSLs are usually multi-purpose research laboratories that conduct diverse studies. Unlike diagnostic or analytical laboratories, whose type of work is usually consistently the same, the projects within a research lab usually change in their nature. In a diagnostic or analytical laboratory, a LIMS can be adopted to manage lab activities once the diagnostic or analytical processes have been determined.[4] Since the information flows in these laboratories usually follow a linear structure, once the LIMS has been installed and connected with automated devices, it can then take its role as the information hub and quality control center of the laboratory.[6] In contrast, BSLs or general research labs comparatively change their experimental activities often, and therefore no single procedure is enough to keep laboratory activities safe and successful. As a result, the LIMS would get downplayed to an ELN or a data reporter attached to a certain piece of automated equipment,[2][8] losing its central position as the information hub of the lab, that is unless we can expand the LIMS' functionality.[7]
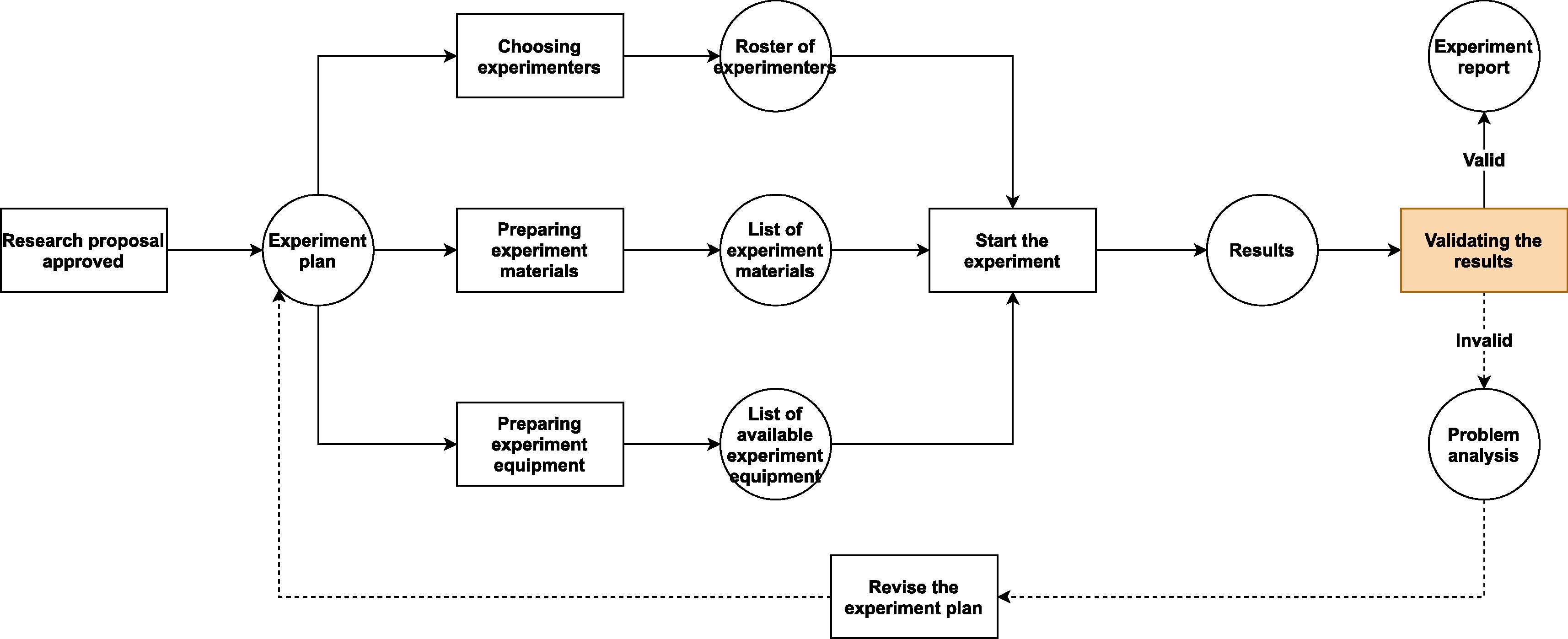
Fortunately, adding the new functions required to make a LIMS adapt to BSL usage is not difficult. All we need to do is to step back a bit and take a look at the bigger picture. From a principal investigator’s point of view, all research activities follow certain pattern (Fig. 1.). If we can expand the system's audit trail and revision control—typically core functions in the LIMS—from managing only "scientific data" to "all data that could affect scientific research," then we should be able to create a system that is able to oversee all research projects in a laboratory. Each of these projects contains three aspects to be managed: the experimenters, the experiment materials, and the experiment equipment and facilities. All of these aspects are affected by the details of the specific project, and the quality of preparation for these aspects will predetermine whether the project will go well or not.
|
Secondly, in regular biological labs, the lab director or other person in charge may do an inspection of the experimenters, the experiment materials, and the experiment equipment and facilities at the beginning of the project. However, this kind of inspection is mostly voluntary and rigid, and the inspected contents are usually arbitrary. There is no mechanism to secure compliance with certain operational rules before and during the project, since it lacks nationally or institutionally enforced laws or regulations. As a result, few lab workers and principal investigators would attempt to make sure that everything that would be involved in the project is well prepared before they start the first experiment; instead, more would try to get started first as soon as the basic conditions of the project has been met, and the complete fulfilment of the experimental conditions would be considered to be a parallel task. This mode might work in some of the regular research labs, but it should be avoided by every BSL.
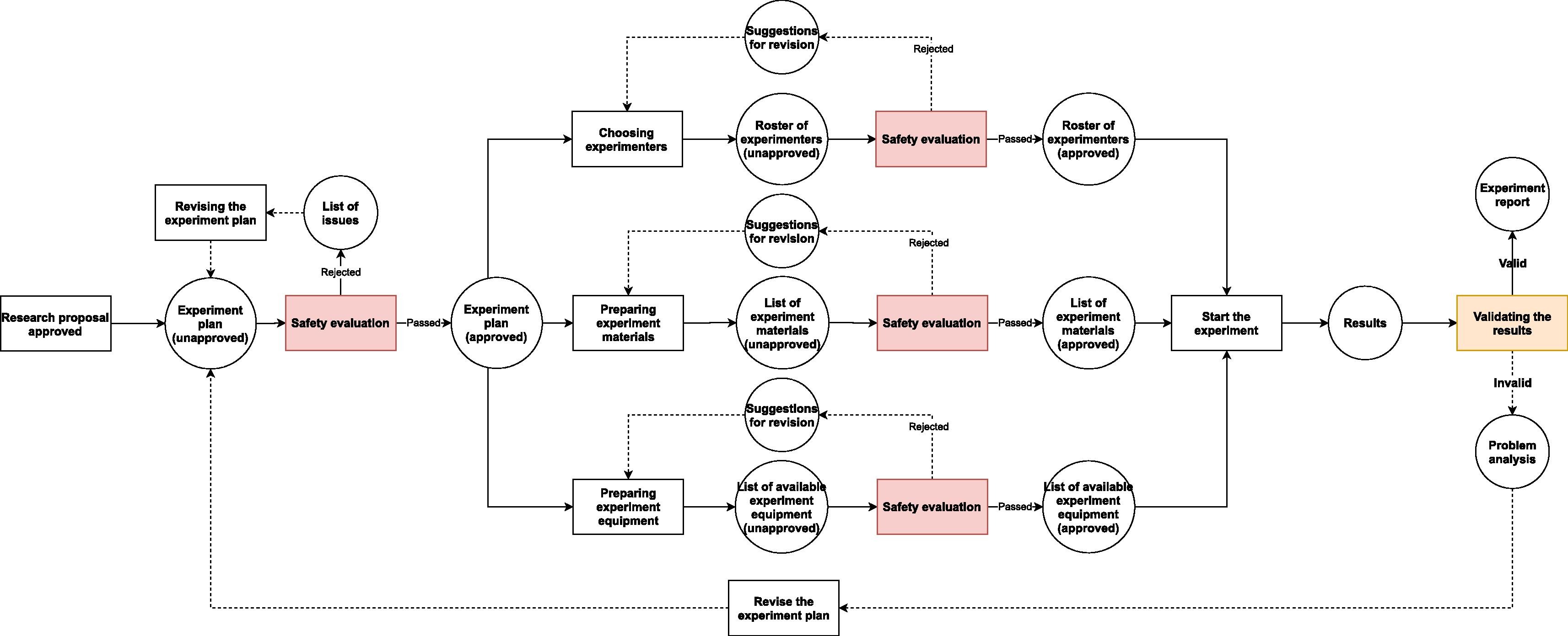
In regular biological labs, since contagious materials or other potentially dangerous self-propagating materials (e.g., gene drive materials) are excluded from experiments, the causes of accidents are usually due to non-biological factors. These factors may lead to very serious consequences, but the range of their influence is mostly limited to the lab itself and its workers. For instance, centrifugation is one of the common activities that can lead to severe injuries or even death of people.[12] Nonetheless, the damage caused by centrifuge failure can hardly go beyond the neighboring rooms where the centrifuge is located and usually only has short-term detrimental effect. In contrast, the leakage of untreated contagious materials from BSLs can lead to massive infection far beyond laboratory workers and last as long as the chain of transmission has not been broken. Even a leakage from a teaching lab of low biosafety level may cause infection in a large community.[13] Hence, BSLs need extra safety measures to counter the increased risk and lower the rate of accidents—especially infection-related accidents—to the minimum (Fig. 2.).
However, there are several potential downsides for doing so. First, adding extra authorization and inspection processes will cost scientists extra time. The increase in the time that they have to spend on non-research affairs will lower their efficiency and subsequently reduce the project's output. Second, humans are prone to errors and thereby can make more mistakes when they are more burdened. Although the aim of extra safety regulations and rules is to lower risk, the increased amount of precautions also adds to the burden of both research staff and biosafety managing staff, making them prone to becoming exhausted by the extra safety information that they need to deal with. This reduction in mental and physical faculties is often a cause of laboratory accidents.[14] Therefore, we need a tireless worker to support human activities, lowering the work load and errors of humans. And that is exactly what a BSL-LIMS can offer.
|
Because the workflows in a research BSL (Fig. 2.) are similar to the ones in a regular multi-purpose biological laboratory (Fig. 1), only with some additional safety checkpoints, the BSL-LIMS can refer to some well-recognized standards (e.g., the International Organization for Standardization [ISO] and Clinical & Labs Standards Institute [CLSI]) and/or previous studies[2][3][4][7][15] to design the modules that are in common to all biological labs (e.g., project-template design, experiment scheduling, sample management, experimental data collection, results analysis and reporting, etc.). In contrast, for the processes that are unique to the BSL, there is no previous standard or study that can be used as examples. Thus, we have to create new modules or update existing LIMS modules to satisfy the requirements of safety information management, based on institutional, national, or international biosafety guidelines.[11][16][17]
Project management can be divided into two parts, based on its effective stage.
The safety modules for project preparation
After a project proposal has been reviewed and passed by the relevant committee(s), and gets funded, the researchers have to turn the contents that they outlined in the proposal into a more detailed and feasible plan. To manage different types of research activities, the LIMS has to be highly flexible and support user configuration; to limit the complexity of such a LIMS, the system needs to be modularized with customizable modules or reusable templates.[7][18] If this is a project that requires BSL usage, then the relevant biosafety databases and safeguard mechanisms are necessary as well. Since most laboratory texts nowadays are stored in a digital form, it is possible to automatically recognize and extract some biosafety related information (e.g., certain words, phrases, etc.) within the texts by using special word processers and relevant databases. The reviewers or biosafety officers can use the extracted information to classify the research projects before giving any further examination, or, if the laboratory texts are all standardized and machine-validated, the computer may be able to do the classification instead of humans, and/or directly warn the researchers about the flaws of their research plan when they try to submit it.[7]
In this module, the biosafety information databases are the cornerstones that determine the upper limit of data automation, though the algorithms also matter. The specific set of databases that are linked to a BSL-LIMS will be determined by the need of the BSL and its research contents, but the types of databases may be more or less the same in all BSLs. The common databases include:
- Microbial information database: The biosafety information of the microbes (especially pathogens) that will be involved in the project directly determines the biosafety level that is required to undertake the project. In terms of pathogenic microorganisms, many countries have established their own pathogen information databases to guide the related studies[19][20], while a few have also made legal policies to regulate non-pathogenic organisms of potential danger.[21][22] Labs and institutions should maintain awareness of local, regional, and national biosafety guidelines or policies, and follow these guidelines and policies all the time. Therefore, whenever such governmental databases exist, labs and institutions should always refer to them if they need to build their own microbial information databases. These databases will help laboratory staff to assess the potential risks of a certain project and provide suggestions on whether and how to undertake it. For all known pathogens or other type of dangerous microbes, the database should include as much information on their pathogenicity or other potentially harmful mechanisms as possible. The research staff and biosafety officers can only make a sensible judgement on the project if they know enough about the type of danger that a microbe poses and its causal mechanisms.[23] For the microbes whose risks are unknown, the maximum containment laboratory (BLS-4) should be used, and some operations that could increase the potential risk should be avoided. The BSL-LIMS should also contain a reporting function (in some cases mandated by national laws) that can allow individual users to report infectious incidents or newly discovered biological risks. Only then can the national databases of biological risks effectively collect new biosafety information, be continuously updated, and provide support to labs in a large area.[24] Theoretically, the national database on dangerous microbes should be open to as many researchers as possible via the internet, allowing as many stakeholders as possible to utilize and contribute to the system. However, due to the serious consequences that could be caused by the malicious use of this information, every country or region should decide which open level is most appropriate based on their own security situation.
- Technical and methodological information database: The infection of a human being is a complex process. It not only concerns the infectious dose (e.g., ID50), but also the state of the contagious material (e.g., DNA or RNA, inactivated strain, genetically modified strain, etc.), and the actual dose of exposure.[11][25] Hence, the actual level of risk can only be assessed after considering both the pathogenicity of the microbe and the experimental methods. The final risk level of an experiment can be higher or lower than the risk level of the pathogen, depending on the specific experimental method that will be used. A well-indexed technical and methodological information database powered by a search engine can be used as a reference system when the researchers are preparing the protocols. And once they submit it to the laboratory managing staff, a word processor can compare the keywords in the database to what it finds in the context, automatically highlighting or warning the reviewers about the parts that may change the risk level.
- Laboratory staff information database: Laboratory staff can be divided into three main categories based on their duties or types of work: research staff, biosafety staff, and logistics staff. Each can be subdivided furthermore based on their skills, positions, or qualifications. A database that contains position, health, reliability, and skill information on laboratory staff is essential for laboratory management. The details of this part will be discussed further in the section "Personnel administration."
- Experimental material information database: A project usually consumes a large amount of experimental materials. Sufficient and high-quality materials are not only the assurance of a successful study but also one of the preconditions for laboratory safety. The details of how to manage this information will be covered in the section "Experimental materials management."
- Equipment information database: Equipment is able to protect experimenters but can be a source of risk as well if being used inappropriately. We will discuss how to manage information related to equipment in order to improve the safety of equipment usage in the section "Equipment management."
The safety modules for ongoing projects
Everything that exits in a biosafety lab, including human beings, may contain biohazards. To protect lab workers and the environment, standard operating procedures (SOPs) and safety guidelines should be followed when experimenters are working. Nonetheless, humans are error-prone and always have a propensity to explore the safety margin in the system to improve efficiency, due to all kinds of pressures.[26] As a result, a safety control mechanism for ongoing projects in BSLs is required to prevent incidents from occurring or being exacerbated. The mechanism usually includes at least an electronic surveillance and detection system, a building automation system with sites that can be remotely controlled, and a health surveillance program for all lab staff. Currently, the lab safety staff’s stewardship is the only additional force to safeguard ongoing projects. Once the research staff have started an experiment, they have to rely on their own good safety practices to keep everything on the right track. The safety officers may frequently monitor the operations of experimenters but only communicate with them when they sense a major risk. In addition, since the focus of experimenters is often not on safety, and safety staff can not always be in the working area monitoring every single move of experimenters, there is generally a lag between the occurrence of a risk and the human detection of it. This reality calls for a better safety warning mechanism that is capable of prevention and early detection of incidents.
Automatically triggered alarms have been widely used in devices with physical or chemical safety thresholds. The alarms or warning devices can warn the related laboratory staff promptly as soon as a potential risk is detected. Thus the lab staff would have enough time to react and prevent accidents from occurring or limit the damage of them. However, most biological incidents are not caused by a single event[26], and biological risks usually lack threshold values.[23] Therefore, this warning mechanism based on continuous monitoring of certain thresholds (e.g., a room's atmospheric pressure, chemical concentration in a closed area, direction of airflow, etc.) are not good enough maintain the overall safety of BSLs. For the risks caused by complex biological operations, this system can do little to help.
A more practical safeguarding mechanism for biohazard control would be a node-based system, in which the experimental information is assembled and submitted to the control node, and machines and/or humans analyze the information and decide if the next action is safe to be taken. Although a LIMS used in diagnostic/analytical labs and research labs has some quality control mechanism to prevent invalid inputs into the next node[4][7], the LIMS is still operated in linear workflow and dealing with single-source data validation. Moreover, since the LIMS is built for linear processes, the results of validation would only go back to the experimenters. The BSL demands the LIMS to be able to validate results from multiple sources, as well as notify safety staff about any sign of risks. Therefore, the control node in BSL-LIMS should be able to automatically validate results from different sources and synthesize the results, and instantly notify both experimenters and safety staff about any anomaly.
Contagious materials and lab workers are the major focuses in biosafety control. As a result, the continuous tracking of their movement would help improve the general safety of the lab or give investigators better insight in accident analysis. For these reasons, the safety module of BSL-LIMS should be connected with certain proximity detectors as well. These detectors are able to monitor human behaviors and/or the movement of important biological samples. Since most accidents occur during the interaction between lab workers and contagious materials, operations involving such kind of interactions deserve special attention and should be monitored as much as possible. The tracking of samples can be realized by physically tagging their containers with whatever method can be turned into electronic signals. For example, scientists have successfully used radio frequency identification (RFID) for item tracking in chemical laboratories.[27] The continuous tracking of humans is more complicated but not impossible. Due to the health concern and large size of human beings, the signal generated by an attached emitter needs to be safe for the human body but strong enough to be read by a receiver. An alternative method is to give experimenters visual labels that can be recognized by a recognition system through cameras. Though direct biometric recognition is difficult in BSLs due to the body coverage by personal protective equipment (PPE), experimenters can be distinguished indirectly by pre-designated visual labels. A color labelling system might be one of the most effective methods to tell the identities of experimenters. For example, every experimenter would be assigned a specific color. The experimenter will always wear the caps or gowns in this color in the lab. With the information prerecorded in a database, their identity can be easily confirmed automatically when the color is captured by a video camera.
Nevertheless, the modules described above merely provide a tracking system that is good for record keeping yet still depend on the vigilance of safety staff to perceive abnormal behaviors and prevent accidents. From this point of view, the movement tracking system is only the first step towards a real automatic accident prevention system.[27] In the future, digital indicators and input terminals should be encompassed into the system together with proximity detectors. Since the indicators and input terminals installed at specific control sites, such as the centrifuges and exits, will automatically react to experimenters’ approach and showing them necessary information, experimenters will be able to receive guidance on their protocol and input data from anywhere in the lab. And the overall safety of the lab should be improved.
Personnel administration
Human workers are at the center of all stages of scientific research activities. They are the main target that we want to protect but also a major cause of incidents in biosafety labs.[14][25] The laboratory staff, especially those who work with pathogens, has a much higher rate of acquiring certain infectious diseases, compared to non-laboratory personnel.[28] Therefore, the effective and efficient administration of laboratory staff is one of the essential parts of maintaining biological safety and security inside and outside of the BSL.
Distinguished by their roles in biosafety laboratories, personnel can generally be divided into three categories:
- Research staff: People in this group are the true users of the laboratory. They make up the majority of laboratory staff, but their population composition can change dynamically because many of them may be students. Since they are frequently in close contact with contagious materials, the safety and health conditions of these people must be examined as often and as rigorously as possible.[23][29] Information relating to research staff’s skill level and health condition (which includes both physical and mental health conditions) should be stored in a database whose contents can be called by the BSL-LIMS. The database needs to be updated frequently, not only by biosafety officers or BSL directors but also by human resource staff, as well as the research staff themselves. Medical history should be attached to research staff's other information for lab management to review. Adjustments must be made when something suggests a person will not be suitable for the current job any longer, which applies to BSL staff in other categories as well. The BSL-LIMS should warn the person in charge when a change in staff information has been made. The alert should not be deactivated until the issue is resolved, which resembles the issue submission system in many online custom support systems. In general, the valid period and access level of an authorization for a research staff are determined by the contents and period of their project.
- Biosafety managing staff: The number of biosafety staff is usually quite small compared to research staff, but their importance can not be overemphasized. As the overseers of the BSL, they are the last line of defense against laboratory accidents. All safety officers who work for BSLs that are above safety level 2 need (usually state) admitted qualifications, showing they have received enough training to direct BSLs of that level. The directors, and sometimes technicians, may be the only safety officers in basic BSLs (BSL-1 and BSL-2), whilst containment labs (BSL-3 and BSL-4) demand a specific team to take care of biosafety matters. Biosafety managing staff are usually permanent employees of the institution. Theoretically, they have the highest authority when dealing with biosafety-related matters. Hence, their qualification and reliability should be inspected periodically, ensuring that they are trustworthy and able to rise to the challenges inherent to the position.
- Support staff: Support staff are responsible for the periodic maintenance of the laboratory facilities, equipment, and the lab construction itself.[11] They affect the extent of safety and efficiency of a laboratory, even though they may not spend as much time in the lab as personnel in the other two categories. The administration of internal engineers and/or animal maintenance staff who are permanent or long-term employees of the institution will be similar to the administration of research staff. If the cleaning services need to be done by specific cleaning staff other than the research and biosafety staff, then it is important to ensure that they have the appropriate qualification, especially when they are responsible for waste disposal and transportation. The qualification and identity of external support staff should be carefully examined by the safety officers, and external support staff should only perform their job under the supervision of safety officers.
Administration of personnel in BSLs requires clarity of job responsibilities and detailed information on each current/potential worker. The access level to a lab is always dependent on the person’s position and specific job. The laboratory director can only recruit or assign a specific type of job to a lab worker if the recruit meets the specific requirements for that job. These requirements are usually listed in the local and/or national laws and regulations, and enhanced by the institution’s own rules.[11][16][23] The training and authorization of most BSL staff follows a hierarchical structure where staff have to finish the training and work in a lower level BSL for a period of time before they can be allowed to enter a higher level BSL. This structure works for each category of personnel. In addition to professional skills and physical health, increased attention has been called to the reliability and mental health of lab workers.[10][14][30] Since biosafety and biosecurity are never a standalone matter, institutions with containment BSLs should work with their local and national government to come up with a feasible reliability program in order to limit the risk that is posed by unsuitable job assignments.
The BSL-LIMS is able to help with the administration of laboratory staff, especially the management of research staff, through automatic filtering of their information. The BSL’s access control firstly depends on the biosafety level of the lab, and then the contents of the specific project. A “machine-human” cooperated process of access authorization can be established. Whenever an application for BSL usage is submitted, the computer will evaluate the applicant's conditions first according to their information in the database, comparing it with the requirements for using that BSL. Then, the application will move to the managing staff for further review if those requirements are satisfied, or it will go back to the applicant with feedback on which condition(s) are or are not fulfilled. The system will repeat this process for a second time with refined requirements of the specific project. By using this hybrid “machine-human” co-evaluation model, human labor can be saved and human error can be reduced.
Experimental materials management
No research can be carried out without experimental materials, though the improper use of experimental materials can pose a major threat to the safety of experimenters and the environment. The general principle of sample management in BSLs is to limit access to the contagious samples and keep a good record of its usage.[31] However, the contagious sample is not the only thing that can inflict risks upon experimenters and environment. Other types of materials—including experimental animals—with poor quality can also put the experimenters at risk. For example, in 2011, a major incident of laboratory-acquired infection (LAI) of Brucella spp. occurred in the Northeast Agriculture University, China, which ended up with 28 people being infected.[32][33] The incident was caused for multiple reasons, but the source of the Brucella bacteria was some uninspected goats used in dissection.
In order to reduce the problems caused by experimental materials, it is important to set up strict rules on the purchase, storage, and usage of them.[6] Though many commercial LIMS have already provided functions related to inventory management, the aim of such modules is to keep the supply chain in the lab uninterrupted. To fulfill the safety requirements of a BSL, the LIMS needs to also be equipped with some safety control mechanism.
The upgraded module in a BSL-LIMS emphasizes the quality control of the experimental materials. Users’ purchase behavior can be limited by enabling a white or black list of suppliers, and a relevant certifications or/and safety data sheet (SDS) should always be provided and uploaded to the material information database. The laboratory staff should keep good records on all of the materials, having their quality indicators (e.g., current state, shelf life, suitable purpose, etc.) in the database. For the materials that could lead to major accidents, e.g. experimental animals or lysis buffers[34], the local testing results and evidence of validity must be submitted to biosafety officers and stored in the corresponding database before further approval can be granted.
Last but not least, the BSL-LIMS should have a tracking system of all biohazardous waste, including information on the method of decontamination, the disposal time, and at least one emergency contact.
Equipment management
Laboratory equipment can be divided into two categories, according to their expected working period: the permanent or durable equipment or facilities, e.g., centrifuges and biosafety cabinets; and non-durable equipment, e.g., personal protective equipment (PPE), disposable pipets, and Petri dishes. Since non-durable equipment are only good for one-time usage, which is similar to experimental materials, their management can be performed in a manner similar to experimental materials. On the other hand, the durable equipment needs to serve the lab for a period of time and be used now and then. Hence, the equipment has to be well understood and well maintained for the laboratory’s safety.
While the experimental materials have their safety data sheets to provide basic safety information, all lab equipment, including non-durables, should also come with similar information. This information, along with the other biosafety related information, can then be provided to the research staff through a client portal to the BSL-LIMS so that staff will be able to learn which specific lab may satisfy their safety demands. The maintenance information on lab equipment should also be included in this database, and periodic maintenance alerts can be set up to remind the corresponding staff to maintain the equipment, or ban the usage of the equipment before it has been maintained. Additionally, because of the busy schedule and special security concern of containment BSLs, a maintenance schedule is always welcome for managing laboratory activities, and an equipment information database that can be filtered by different conditions will surely help to create such a schedule.
Beyond the basics
Laboratory safety is a dynamic process that requires continuous inspection and updating rather than a one-and-done activity. Laboratory directors and staff must be aware of that and adjust to any new challenges while maintaining a high level of alertness towards all the known risks. These challenges may come from newly discovered pathogens, as well as the application of new technologies (e.g., gene drive).[22][35][36] The BSL-LIMS needs to cope with the changes in every aspect of a lab, and as such the system must be easily expandable. However, a highly expandable and flexible system is frequently equal to a highly complex one, making every change in the system unnecessarily time-consuming.[18] Determining the trade off between the flexibility and the complexity of the system will be a challenging job for software engineers and product managers. In addition, proof-of-concept systems need to be established before the application of any BSL-LIMS in large scale. Even though the system may be designed to fit in different niches, unexpected issues can always be found in the trials.[10] Because of the extra safety risks related to improper management of biosafety information, it is always necessary to have a newly developed BSL-LIMS tested on a small scale for enough time before dispensing it to more users.
Another aspect that should not be overlooked is the data security of the BSL-LIMS. Since the databases used by a BSL-LIMS may be public, internal, or confidential, the system must be able to distinguish the different type of databases and prevent the leakage of protected data. From this point of view, it might be best to put the BSL-LIMS in a local area network (LAN), isolating it from all possible unauthorized access. If it is impossible, then an intermediate connection like the virtual private network should be set up to encrypt data transmission between internal and external databases, and prevent data leakage.
Acknowledgements
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- ↑ Gibbon, Gerst A. (1 May 1996). "A brief history of LIMS" (in en). Laboratory Automation & Information Management 32 (1): 1–5. doi:10.1016/1381-141X(95)00024-K. https://linkinghub.elsevier.com/retrieve/pii/1381141X9500024K.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Goodman, N.; Rozen, S.; Stein, L. D. (1998). "The LabFlow system for workflow management in large scale biology research laboratories". Proceedings. International Conference on Intelligent Systems for Molecular Biology 6: 69–77. ISSN 1553-0833. PMID 9783211. https://pubmed.ncbi.nlm.nih.gov/9783211.
- ↑ 3.0 3.1 3.2 3.3 Ludäscher, Bertram; Altintas, Ilkay; Berkley, Chad; Higgins, Dan; Jaeger, Efrat; Jones, Matthew; Lee, Edward A.; Tao, Jing et al. (25 August 2006). "Scientific workflow management and the Kepler system" (in en). Concurrency and Computation: Practice and Experience 18 (10): 1039–1065. doi:10.1002/cpe.994. ISSN 1532-0626. https://onlinelibrary.wiley.com/doi/10.1002/cpe.994.
- ↑ 4.0 4.1 4.2 4.3 4.4 Skobelev, D. O.; Zaytseva, T. M.; Kozlov, A. D.; Perepelitsa, V. L.; Makarova, A. S. (1 January 2011). "Laboratory information management systems in the work of the analytic laboratory" (in en). Measurement Techniques 53 (10): 1182–1189. doi:10.1007/s11018-011-9638-7. ISSN 0543-1972. http://link.springer.com/10.1007/s11018-011-9638-7.
- ↑ McDonald, Michael J.; Rice, Daniel P.; Desai, Michael M. (1 March 2016). "Sex speeds adaptation by altering the dynamics of molecular evolution" (in en). Nature 531 (7593): 233–236. doi:10.1038/nature17143. ISSN 0028-0836. PMC PMC4855304. PMID 26909573. http://www.nature.com/articles/nature17143.
- ↑ 6.0 6.1 6.2 Organization, World Health; World Health Organization, Centers for Disease Control and Prevention (U.S.); World Health Organization; Clinical and Laboratory Standards Institute (2011). Laboratory Quality Management System. Geneva: World Health Organization. ISBN 978-92-4-068857-5. OCLC 1162457940. https://public.ebookcentral.proquest.com/choice/publicfullrecord.aspx?p=1142071.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 Melo, Alexandre; Faria-Campos, Alessandra; DeLaat, Daiane; Keller, Rodrigo; Abreu, Vinícius; Campos, Sérgio (2010). "SIGLa: an adaptable LIMS for multiple laboratories" (in en). BMC Genomics 11 (Suppl 5): S8. doi:10.1186/1471-2164-11-S5-S8. ISSN 1471-2164. PMC PMC3045801. PMID 21210974. http://bmcgenomics.biomedcentral.com/articles/10.1186/1471-2164-11-S5-S8.
- ↑ 8.0 8.1 IGI Testing Consortium; Amen, Alexandra M.; Barry, Kerrie W.; Boyle, John M.; Brook, Cara E.; Choo, Seunga; Cornmesser, L. T.; Dilworth, David J. et al. (1 July 2020). "Blueprint for a pop-up SARS-CoV-2 testing lab" (in en). Nature Biotechnology 38 (7): 791–797. doi:10.1038/s41587-020-0583-3. ISSN 1087-0156. http://www.nature.com/articles/s41587-020-0583-3.
- ↑ Stuart, Ralph; Stewart, James; Herrick, Robert (29 March 2019), Mansdorf, S. Z., ed., "INFORMATION RESOURCES FOR OCCUPATIONAL SAFETY AND HEALTH PROFESSIONALS" (in en), Handbook of Occupational Safety and Health (Hoboken, NJ, USA: John Wiley & Sons, Inc.): 37–48, doi:10.1002/9781119581482.ch2, ISBN 978-1-119-58148-2, https://onlinelibrary.wiley.com/doi/10.1002/9781119581482.ch2. Retrieved 2021-10-22
- ↑ 10.0 10.1 10.2 Trevan, Tim (1 November 2015). "Biological research: Rethink biosafety" (in en). Nature 527 (7577): 155–158. doi:10.1038/527155a. ISSN 0028-0836. http://www.nature.com/articles/527155a.
- ↑ 11.0 11.1 11.2 11.3 11.4 World Health Organization, ed. (2004). Laboratory biosafety manual (3rd ed ed.). Geneva: World Health Organization. ISBN 978-92-4-154650-8. OCLC ocm57658996. https://www.worldcat.org/title/mediawiki/oclc/ocm57658996.
- ↑ Kolff, J.; Ankney, R. N.; Wurzel, D.; Devineni, R. (1 September 1996). "Centrifugal pump failures". The Journal of Extra-Corporeal Technology 28 (3): 118–122. ISSN 0022-1058. PMID 10163498. https://pubmed.ncbi.nlm.nih.gov/10163498.
- ↑ Emmert, Elizabeth A. B.; the ASM Task Committee on Laboratory Biosafety (1 January 2013). "Biosafety Guidelines for Handling Microorganisms in the Teaching Laboratory: Development and Rationale" (in en). Journal of Microbiology & Biology Education 14 (1): 78–83. doi:10.1128/jmbe.v14i1.531. ISSN 1935-7877. PMC PMC3706168. PMID 23858356. https://journals.asm.org/doi/10.1128/jmbe.v14i1.531.
- ↑ 14.0 14.1 14.2 Wurtz, N.; Papa, A.; Hukic, M.; Di Caro, A.; Leparc-Goffart, I.; Leroy, E.; Landini, M. P.; Sekeyova, Z. et al. (1 August 2016). "Survey of laboratory-acquired infections around the world in biosafety level 3 and 4 laboratories" (in en). European Journal of Clinical Microbiology & Infectious Diseases 35 (8): 1247–1258. doi:10.1007/s10096-016-2657-1. ISSN 0934-9723. PMC PMC7088173. PMID 27234593. http://link.springer.com/10.1007/s10096-016-2657-1.
- ↑ ASTM International (2018). "ASTM E1578 - 18 Standard Guide for Laboratory Informatics". ASTM International. https://www.astm.org/Standards/E1578.htm.
- ↑ 16.0 16.1 AQSIQ (26 December 2008). "GB 19489-2008 Laboratories - General Requirements for Biosafety (English Version)". Code of China. https://www.codeofchina.com/standard/GB19489-2008.html.
- ↑ State Council of China (6 February 2016). "Regulation on the Bio-safety Management of Pathogenic Microbe Labs - Revised". lawinfochina.com. http://www.lawinfochina.com/display.aspx?lib=law&id=3849&CGid=.
- ↑ 18.0 18.1 Craig, Thomas; Holland, Richard; D’Amore, Rosalinda; Johnson, James R.; McCue, Hannah V.; West, Anthony; Zulkower, Valentin; Tekotte, Hille et al. (15 December 2017). "Leaf LIMS: A Flexible Laboratory Information Management System with a Synthetic Biology Focus" (in en). ACS Synthetic Biology 6 (12): 2273–2280. doi:10.1021/acssynbio.7b00212. ISSN 2161-5063. https://pubs.acs.org/doi/10.1021/acssynbio.7b00212.
- ↑ Ministry of Health of the People's Republic of China (2006). "卫生部关于印发《人间传染的病原微生物名录》的通知". National Health Commission of the People's Republic of China. http://www.nhc.gov.cn/wjw/gfxwj/201304/64601962954745c1929e814462d0746c.shtml.
- ↑ Public Health Agency of Canada (2021). "Pathogen Safety Data Sheets". Government of Canada. https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment.html.
- ↑ National Academies of Sciences, Engineering, and Medicine (U.S.), ed. (2016). Gene drives on the horizon: advancing science, navigating uncertainty, and aligning research with public values. Washington, D.C: The National Academies Press. ISBN 978-0-309-43787-5. OCLC 950893787. https://www.worldcat.org/title/mediawiki/oclc/950893787.
- ↑ 22.0 22.1 Lunshof, Jeantine E.; Birnbaum, Angela (1 September 2017). "Adaptive Risk Management of Gene Drive Experiments: Biosafety, Biosecurity, and Ethics" (in en). Applied Biosafety 22 (3): 97–103. doi:10.1177/1535676017721488. ISSN 1535-6760. http://journals.sagepub.com/doi/10.1177/1535676017721488.
- ↑ 23.0 23.1 23.2 23.3 Macher, Janet M.; Gold, Deborah; Cruz, Patricia; Kyle, Jennifer L.; Durrani, Timur S.; Shusterman, Dennis (29 March 2019), Mansdorf, S. Z., ed., "EVALUATION AND MANAGEMENT OF EXPOSURE TO INFECTIOUS AGENTS" (in en), Handbook of Occupational Safety and Health (Hoboken, NJ, USA: John Wiley & Sons, Inc.): 147–197, doi:10.1002/9781119581482.ch6, ISBN 978-1-119-58148-2, https://onlinelibrary.wiley.com/doi/10.1002/9781119581482.ch6. Retrieved 2021-10-25
- ↑ Singh, Kamaljit (1 August 2011). "It's time for a centralized registry of laboratory-acquired infections" (in en). Nature Medicine 17 (8): 919–919. doi:10.1038/nm0811-919. ISSN 1078-8956. http://www.nature.com/articles/nm0811-919.
- ↑ 25.0 25.1 Weinstein, R.A.; Singh, K. (1 July 2009). "Laboratory‐Acquired Infections" (in en). Clinical Infectious Diseases 49 (1): 142–147. doi:10.1086/599104. ISSN 1058-4838. PMC PMC7107998. PMID 19480580. https://academic.oup.com/cid/article-lookup/doi/10.1086/599104.
- ↑ 26.0 26.1 Leveson, Nancy (1 April 2004). "A new accident model for engineering safer systems" (in en). Safety Science 42 (4): 237–270. doi:10.1016/S0925-7535(03)00047-X. https://linkinghub.elsevier.com/retrieve/pii/S092575350300047X.
- ↑ 27.0 27.1 Nezu, Y.; Hayashi, R.; Yamamoto, H. et al. (2019). "Visualization of activity in university laboratory for experimental accidents/incidents prevention using non-empirical approach". Journal of Environment and Safety 10 (2): 45-47. doi:10.11162/daikankyo.E18PROCP46.
- ↑ Skinhoj, P; Soeby, M (1 April 1981). "Viral hepatitis in Danish health care personnel, 1974-78." (in en). Journal of Clinical Pathology 34 (4): 408–411. doi:10.1136/jcp.34.4.408. ISSN 0021-9746. PMC PMC493301. PMID 7240429. http://jcp.bmj.com/cgi/doi/10.1136/jcp.34.4.408.
- ↑ Harding, A. Lynn; Byers, Karen Brandt (2006), Fleming, Diane O.; Hunt, Debra L., eds., "Epidemiology of Laboratory-Associated Infections" (in en), Biological Safety (Washington, DC, USA: ASM Press): 53–77, doi:10.1128/9781555815899.ch4, ISBN 978-1-68367-177-0, http://doi.wiley.com/10.1128/9781555815899.ch4
- ↑ Higgins, Jacki J.; Weaver, Patrick; Fitch, J. Patrick; Johnson, Barbara; Pearl, R. Marene (1 June 2013). "Implementation of a Personnel Reliability Program as a Facilitator of Biosafety and Biosecurity Culture in BSL-3 and BSL-4 Laboratories" (in en). Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science 11 (2): 130–137. doi:10.1089/bsp.2013.0024. ISSN 1538-7135. PMC PMC3689182. PMID 23745523. http://www.liebertpub.com/doi/10.1089/bsp.2013.0024.
- ↑ Asfaw, Yohannes G; Reynolds, Randall; Alderman, Scott; Norton, John N (31 December 2018). "Managing Research Animal Specimens and Laboratory Safety" (in en). ILAR Journal 59 (2): 144–149. doi:10.1093/ilar/ily017. ISSN 1084-2020. https://academic.oup.com/ilarjournal/article/59/2/144/5257453.
- ↑ Nan, Z. (6 September 2011). "东北农大就师生感染布鲁氏菌病致歉". ScienceNet.cn. http://news.sciencenet.cn/sbhtmlnews/2011/9/248521.shtm?id=248521.
- ↑ Song, Langui; Gao, Jiangmei; Wu, Zhongdao (1 April 2021). "Laboratory-acquired infections with Brucella bacteria in China" (in en). Biosafety and Health 3 (2): 101–104. doi:10.1016/j.bsheal.2020.07.010. https://linkinghub.elsevier.com/retrieve/pii/S2590053620300835.
- ↑ Ngo, Kiet A.; Jones, Susan A.; Church, Theresa M.; Fuschino, Meghan E.; George, Kirsten St.; Lamson, Daryl M.; Maffei, Joseph; Kramer, Laura D. et al. (1 June 2017). "Unreliable Inactivation of Viruses by Commonly Used Lysis Buffers" (in en). Applied Biosafety 22 (2): 56–59. doi:10.1177/1535676017703383. ISSN 1535-6760. http://journals.sagepub.com/doi/10.1177/1535676017703383.
- ↑ Akbari, Omar S.; Bellen, Hugo J.; Bier, Ethan; Bullock, Simon L.; Burt, Austin; Church, George M.; Cook, Kevin R.; Duchek, Peter et al. (28 August 2015). "Safeguarding gene drive experiments in the laboratory" (in en). Science 349 (6251): 927–929. doi:10.1126/science.aac7932. ISSN 0036-8075. PMC PMC4692367. PMID 26229113. https://www.science.org/doi/10.1126/science.aac7932.
- ↑ DiCarlo, James E; Chavez, Alejandro; Dietz, Sven L; Esvelt, Kevin M; Church, George M (1 December 2015). "Safeguarding CRISPR-Cas9 gene drives in yeast" (in en). Nature Biotechnology 33 (12): 1250–1255. doi:10.1038/nbt.3412. ISSN 1087-0156. PMC PMC4675690. PMID 26571100. http://www.nature.com/articles/nbt.3412.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. Nothing else was changed in accordance with the NoDerivatives portion of the license.