Validation 4.0 Model for a Digital Transformation of the Life Sciences Industry

Validation 4.0 requires a high level of data integration, increased supply chain visibility and resiliency, and that the right processes, skillset and technology are leveraged. In order to ensure success of Validation 4.0 implementation, organizations need to understand how Validation 4.0 maps to the Product Life Cycle (PLC)…
In order to ensure success of Validation 4.0 implementation, organizations need to understand how Validation 4.0 maps to the Product Life Cycle (PLC) – ensuring that product quality and patient safety are kept to the highest standards.
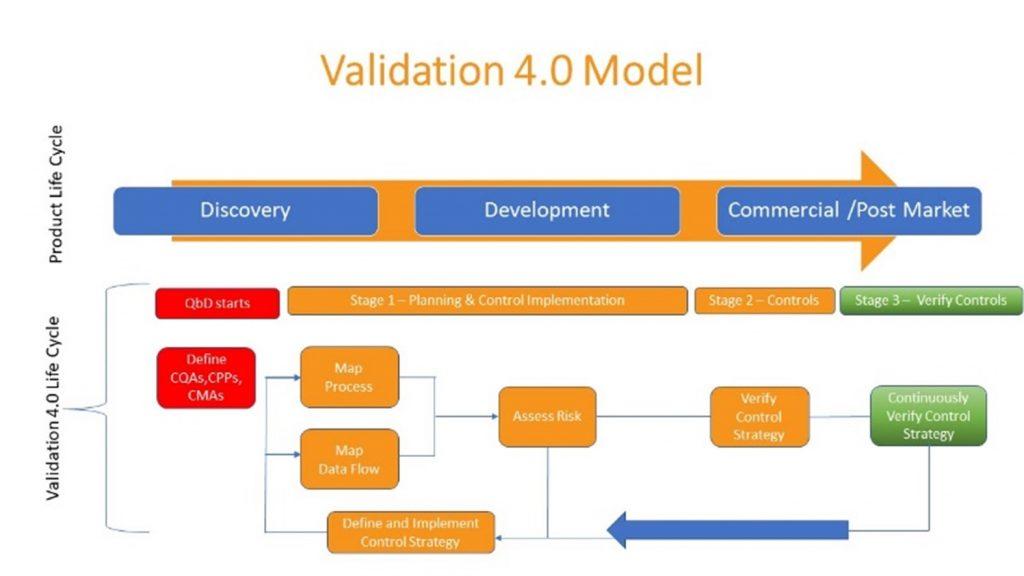
Source: Special Interest Briefing on Validation 4.0: Objectives, Working Model, and Relation to QBD
With that in mind, there is a need for a framework that assists in the transition to Validation 4.0 by showing how it maps to the PLC. A Validation 4.0 model highlighting this mapping was discussed in June of 2021 on an ISPE webinar on this topic. This model is the foundation for incorporating Validation 4.0 into the organization. It reviews the PLC stages along with the inter-dependency of some of the activities across the phases.
Product Life Cycle
PLC goes from Discovery, to Development, to Commercialization, and then to Post Market. The Validation life cycle has corresponding steps that provide data and feedback to ensure success. Quality by Design (QbD) attributes such as the Critical Quality Attributes (CQAs), the Critical Process Parameters (CPPs), the Critical Material Attributed (CMAs), and other key considerations would need to be incorporated into the PLC to provide the required visibility as well as monitoring of the overall process.
Quality By Design
Utilizing digital tools as an enabler across all stages of the validation process provides ready access to critical variables, parameters and requirements as they change throughout the process. It is important to note that QbD is not a signal step of the process but rather an iterative approach we use in order to learn more about our product and processes. With QbD, we accumulate information that assists in better understanding the products and processes so as to improve efficiency and optimization across all stages.
Planning & Control Implementation Stage
Stage 1, in the Validation 4.0 life cycle is the planning & control implementation phase. This maps to the discovery and development stage of PLC. In this stage, the organization’s user requirements are captured through process maps and data flows. This involves a digitization of the process leveraging technology. In this stage, the requirements that provide a better understanding of what the organization needs from a process and products perspective are contextualized. Additionally, a risk based assessment is applied that is associated with the process maps and data flows. This is where the risk is measured as it impacts product quality and patient safety. Criticality and vulnerability of the risk are both key considerations relative to this area.
Controls Stage
The Implementation of Controls is the next stage, Stage 2, in the Validation 4.0 Life Cycle. In this phase, we have digital tools in place to collect, report, and act on critical data in real-time.
Verify Controls Stage
The following stage, Stage 3, is the Verify Controls phase. In this stage, the critical data from the on-going process are continually monitored to provide opportunities for optimization as well as implement any changes to the system based on performance monitoring. The digital monitoring is to ensure that the design of the system to control the process and data is providing an acceptable level of risk. Data is leveraged for continuous improvement and optimization of the process. A continual feedback mechanism is required where the data is collected and analyzed in real-time so that the appropriate action on any discrepancies can be taken.
Summary
In order to successfully incorporate Validation 4.0 into the business, there needs to be an understanding of the Product Life Cycle (PLC). Additionally, the requirements associated with the processes and data need to be digitized so that they can be measured throughout the life cycle. This is required in order to ensure that the product quality and patient safety is kept at the highest level. To do this, there is a need for a Validation 4.0 model that maps to the PLC that can be leveraged to assist in ensuring the monitoring of the data and processes via controls. This model needs to incorporate the appropriate technologies that help to report, control, and improve the validation process across the life cycle. The validation process also needs to be continuously monitored to verify that standards are kept in place.
Why It Matters to You
Validation is the key to ensuring that product quality and patient safety are kept at a high standard. Validation 4.0 takes validation to the next level by ensuring that the processes are digitized and has the proper controls throughout the Product Life Cycle. In this blog we discuss:
- A Validation 4.0 model that assists in controlling product quality and patient safety.
- How the Validation Life Cycle maps to the Product Life Cycle.
- How data, processes, risk, and controls play a part in the Validation 4.0 model.
- Key elements of the Validation Life Cycle to consider to help ensure success.
About Astrix
For over 25 years, Astrix has been a market-leader in dedicated digital transformation & staffing services for science-based businesses. Through our proven laboratory informatics, digital quality & compliance, and scientific staffing services we deliver the highly specialized people, processes, and technology to fundamentally transform how science-based businesses operate. Astrix was founded by scientists to solve the unique challenges which science-based businesses face in the laboratory and beyond. We’re dedicated to helping our clients speed & improve scientific outcomes to help people everywhere.
















