| 08/17/2022 - Iterating a Validated Assay? You Need Effective Change Management  Day-to-day lab operations rely on workflows to manage people, tasks, and systems so that work follows a consistent process every time. Defined workflows help ensure that all the steps are done in the correct order, are free from errors, and meet regulatory requirements. But, representing lab workflows can be difficult. [Read More] Day-to-day lab operations rely on workflows to manage people, tasks, and systems so that work follows a consistent process every time. Defined workflows help ensure that all the steps are done in the correct order, are free from errors, and meet regulatory requirements. But, representing lab workflows can be difficult. [Read More]
08/17/2022 - Reducing Human Error Before and After Lab Testing
08/17/2022 - How Logilab ELN helps organizations to follow GxP Regulations  GxP is a set of regulations and quality guidelines formulated to ensure the safety of life sciences products while maintaining the quality of processes throughout every stage of manufacturing, control, storage, and distribution. The GxP standards were established by the Food and Drug Administration for a range of compliance related activities. [Read More] GxP is a set of regulations and quality guidelines formulated to ensure the safety of life sciences products while maintaining the quality of processes throughout every stage of manufacturing, control, storage, and distribution. The GxP standards were established by the Food and Drug Administration for a range of compliance related activities. [Read More]
08/17/2022 - Best Practices for a Data Migration Plan and Integration of a New Pharmacovigilance System  An effective data migration plan ensures your data is accurate and thorough during the critical transfer phase from the source platform to the destination platform. Once your team is assembled, it is crucial that they focus first on mapping, selecting, preparing, extracting, transforming, and transferring to the new system data that’s of the proper form and quality. [Read More] An effective data migration plan ensures your data is accurate and thorough during the critical transfer phase from the source platform to the destination platform. Once your team is assembled, it is crucial that they focus first on mapping, selecting, preparing, extracting, transforming, and transferring to the new system data that’s of the proper form and quality. [Read More]
08/17/2022 - Discover the Power of ApolloLIMS Backed by CliniSys Group  Earlier this year, ApolloLIMS and HORIZON Lab Systems were acquired by CliniSys Group to grow the organization’s community and public health diagnostics capability. CliniSys is one of the largest providers of laboratory information systems (LIS), order entry and result consultation, and public health solutions in disease surveillance and outbreak management across the United Kingdom, Europe, and United States. [Read More] Earlier this year, ApolloLIMS and HORIZON Lab Systems were acquired by CliniSys Group to grow the organization’s community and public health diagnostics capability. CliniSys is one of the largest providers of laboratory information systems (LIS), order entry and result consultation, and public health solutions in disease surveillance and outbreak management across the United Kingdom, Europe, and United States. [Read More]
08/17/2022 - Navigating LabWare’s Message Engine Template 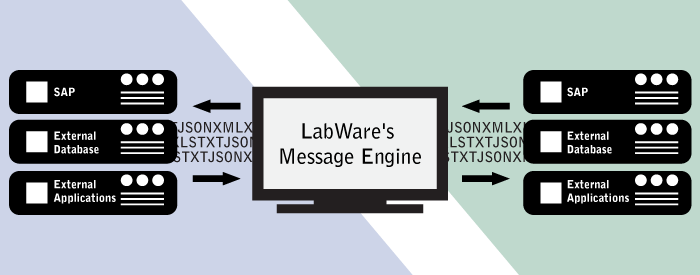 Does your LabWare implementation connect to external applications, such as SAP, another LIMS solution, or an external database? Are there multiple interfaces between LabWare and those external applications? If so, your LabWare implementation will greatly benefit from the Message Engine Template, as it is LabWare’s standardized and validated way to connect with external systems. In this post, we’ll learn about how LabWare’s Message Engine works... [Read More] Does your LabWare implementation connect to external applications, such as SAP, another LIMS solution, or an external database? Are there multiple interfaces between LabWare and those external applications? If so, your LabWare implementation will greatly benefit from the Message Engine Template, as it is LabWare’s standardized and validated way to connect with external systems. In this post, we’ll learn about how LabWare’s Message Engine works... [Read More]
|