An assessment of heavy metal contaminants related to cannabis-based products in the South African market
| Full article title | An assessment of heavy metal contaminants related to cannabis-based products in the South African market |
|---|---|
| Journal | Forensic Science International Reports |
| Author(s) | Viviers, Hendrik J.; Petzer, Anél; Gordon, Richard |
| Author affiliation(s) | National Analytical Forensic Services, North West University, South African Medical Research Council |
| Primary contact | Email: henrick at nafs dot co dot za |
| Year published | 2021 |
| Volume and issue | 4 |
| Article # | 100224 |
| DOI | 10.1016/j.fsir.2021.100224 |
| ISSN | 2665-9107 |
| Distribution license | Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International |
| Website | https://www.sciencedirect.com/science/article/pii/S2665910721000554 |
| Download | https://www.sciencedirect.com/science/article/pii/S2665910721000554/pdfft (PDF) |
Abstract
South African cannabis-based products that were submitted to a private laboratory for the determination of heavy metals residues were analyzed. The presence of each heavy metal residue was determined in order to establish which residues are most prevalent in samples. Two specifications were considered for both oral as well as inhalation limits: United States Pharmacopeia (USP) <232>/<233> and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q3D. To date, no data of this kind exist in South Africa specifically relating to cannabis-based medicinal, recreational, or complementary products. A total of 310 samples were analyzed in duplicate and are reported in an anonymized format. The submitted samples were divided into different category classifications and grouped according to relevance for oral or inhalation specification. The results showed an alarming 15% sample failure rate, compared to the oral specification limit, and a 44% failure rate, compared to the inhalation specification limit. It is of the utmost importance for manufacturers to have the appropriate quality control regimes in place, especially for heavy metal residues, in South Africa. Furthermore, it is imperative to ensure regulation is enforced and the South African public is educated about the risks associated with using these products.
Keywords: cannabis, heavy metals, regulation, residues, South Africa
Introduction
In the cannabis industry, be it medicinal or recreational, there is an abundance of control measures in place to ensure product safety and efficacy.[1] Contamination of Cannabis plants with toxic heavy metals such as arsenic, cadmium, lead etc. can result from numerous origins. Sources of contamination include environmental pollution such as emissions from factories and automobiles, contaminated water, some pesticides, and naturally occurring metals in soil and fertilizers.[2] The contamination of the herbal material ultimately leads to contamination of the products during various stages of the manufacturing process.[3][4]
During growth, metals accumulate in the biomass of specific plants.[5] Studies conducted on industrial hemp show that the Cannabis plant bioaccumulates heavy metals from the soil, and thus is readily employed for phytoremediation of contaminated soils.[6] There have also been reported cases of post-processing adulteration of cannabis buds, adding heavy metals to increase the weight of the product to purposely increase the street value.[2]
Pesticides that contain arsenic and mercury as part of their structures were commonly utilized until a few years ago, and they are still employed in some capacity to date. These toxic substances are likely to be present in many foods due to their abundance in nature, and it is important to note that associated ingestion or inhalation of these cannabis products would add to the accumulation of heavy metals consumed by people, even if best practice guidelines are followed.[1]
As a result of the new requirements imposed by the United States Pharmacopeia (USP) <232>/<233>[7], in collaboration with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q3D[8], the detection limits for certain heavy metals have been lowered. Inductively coupled plasma mass spectrometry (ICP-MS) is a recommended technique for detecting heavy metal contamination. Given that technique, low residue limits can be imposed by USP <232>/<233>.[7][9]
Heavy metal residues in pharmaceutical end products, active pharmaceutical ingredients, and excipients need to be controlled and should be at a certain limit for safe human consumption.[9][10] Furthermore, it can be noted in the somewhat unique case of cannabis-based products, that an alternative route of administration of these products does occur, namely inhalation. The pharmacopeial guidelines stipulate three routes of administration, namely parenteral, oral, and inhalation.[7][8] Cannabis-based products are predominantly administered through oral and inhalation pathways.
Heavy metals are classified into different classes according to their toxic potential, with Class 1 (Table 1) being the most dangerous, Class 2 being less toxic, and Class 3 having the highest limits and being the least toxic. This study will focus on Class 1 and 2 metal residues given they present the greatest health risk to consumers.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
It is the aim of this study to analyze a segment of the South African cannabis-based products in circulation and provide a detailed overview of the elemental impurities/heavy metal residues contained in these products. Furthermore, the adherence of these samples to the imposed inhalation and oral limits by the USP and ICH will also be evaluated.[7][8] To date, no data of this kind exist in South Africa. With these data, regulators, medical doctors, and the public can gain a better sense of the dangers currently being faced regarding cannabis-based products in South Africa.
Materials and methods
A Nexion 350D ICP-MS, which was employed for elemental analysis, was obtained from Perkin Elmer corporation (Waltham, Massachusetts, United States). An in-house method was developed for the analysis of these products and validated according to ICH Q2(r1), for linearity, precision, accuracy and stability. A five-point calibration was employed for all analytes and ranged from 0.0018 ppm to 0.5 ppm. All calibration curves exhibited a correlation coefficient (r2) of 0.999 or better. A summary of validation data is listed in Appendix A, Table A5. Certified reference materials (CRMs) were sourced locally, and a custom standard mix was prepared according to the stability of the metals as well as the classes analyzed. The first CRM 250MUL4A contained Cd (10 µg/mL), Pb (10 µg/mL), As (30 µg/mL), and Hg (60 µg/mL) in 5% HNO3. The second CRM 125AG30-HNO3 contained Ag (30 µg/mL) in 5% HCl. The third CRM 250MUL12A contained Tl (1.6 µg/mL), Co (10 µg/mL), Au (20 µg/mL), Ir (20 µg/mL), Pd (20 µg/mL), Pt (20 µg/mL), Rh (20 µg/mL), Ru (20 µg/mL), Os (20 µg/mL), V (20 µg/mL), Se (30 µg/mL), and Ni (40 µg/mL) in 5% HNO3. Samples were evaluated against a pharmacopeial limit imposed by both USP <232>/<233> and ICH Q3D.[7][8] It should be noted that three routes of dose administration are listed in both these guidelines: parenteral, oral, and inhalation. No parenteral samples have been received to date, but oral and inhalation specification limits were considered.
A total of 310 samples were submitted to a South African contract laboratory for analysis. Manufacturers are defined as any type of user, retailer, reseller, producer, or importer of cannabis-based products. Whether these manufacturers maintain the full value chain or only a portion thereof, they are defined as manufacturers for the purpose of this study. Manufacturers may include cultivators of plants, producers of products, importers, resellers, and pharmaceutical manufacturers. Sample data will be presented anonymously. It should be noted that samples were analyzed as received by the laboratory, irrespective of whether plant material was dry or wet. Dry plant material will have a larger portion of elemental impurities as a result of the moisture mass loss during the drying process. The moisture content of the sample may influence results significantly since they are reported in a mass per mass unit. All samples were analyzed in duplicate. Class 1 and 2 heavy metal test panels were analyzed (See Table 1). It should be noted that the majority of samples were cannabis-based products, with very few samples submitted for the analysis of soil and water. Consent was provided to employ the data for research purposes.
The samples were categorized into seven different types and are shown in Table 2, the same as reported by a potency study conducted by the same laboratory.[11]
| ||||||||||||||||||
Approximately 200 mg of each sample was weighed, digested in 10% HNO3 for 1 hour at 100°C and diluted 35 times to a total volume of 7 mL. Internal Standard Y (Yttrium) and In (Indium) was used for matrix interference correction. Since large isolate and extract sample quantities are scarce, a method needed to be developed to be able to employ as small as possible sample quantity while still being able to reach the USP <232>/<233> and ICH Q3D limits. Analysis started with a blank run, then a five-point calibration curve, followed by a control standard every 10 duplicates, to avoid instrumental drift. Sample sets were ended by analyzing a control standard to ensure all samples within a sample set adhered to bias and variation limits as per USP232/ICHQ3D. The data were grouped into two major groups containing categories applicable to either the inhalation limits and/or categories applicable to the oral specification limits as per USP <232>/<233> and ICH Q3D. All categories were included in the oral specification limit, since all samples needed to be compared to a specification. As for the inhalation specification, the extract, liquid, and plant material categories were grouped together for comparison. Since it was not known by the laboratory what the final intended use of the products were, these three categories posed the highest likelihood being dosed in inhalation form. The two major dosage form groups were also subdivided into two different categories:
- Individual heavy metal residues were grouped together, with no relationship to the sample or matrix, in order to determine the number of times this specific metal residue failed to adhere to the USP <232>/<233> and ICH Q3D specification.
- Samples were grouped together to determine the frequency of failed samples. The frequency of failed samples only considered whether any one of the heavy metal residues in the test panel failed.
Lastly, the occurrence of heavy metal residues was determined, without any adherence to a specification limit. This provided valuable data on the metal residues that are predominantly present, in addition to whether they are able to be detected by the test method. Heavy metal residues were noted as either present when it was detected in a sample, or not detected (ND). Limits of detection are listed in Appendix A, Tables A3 and A5.
Results
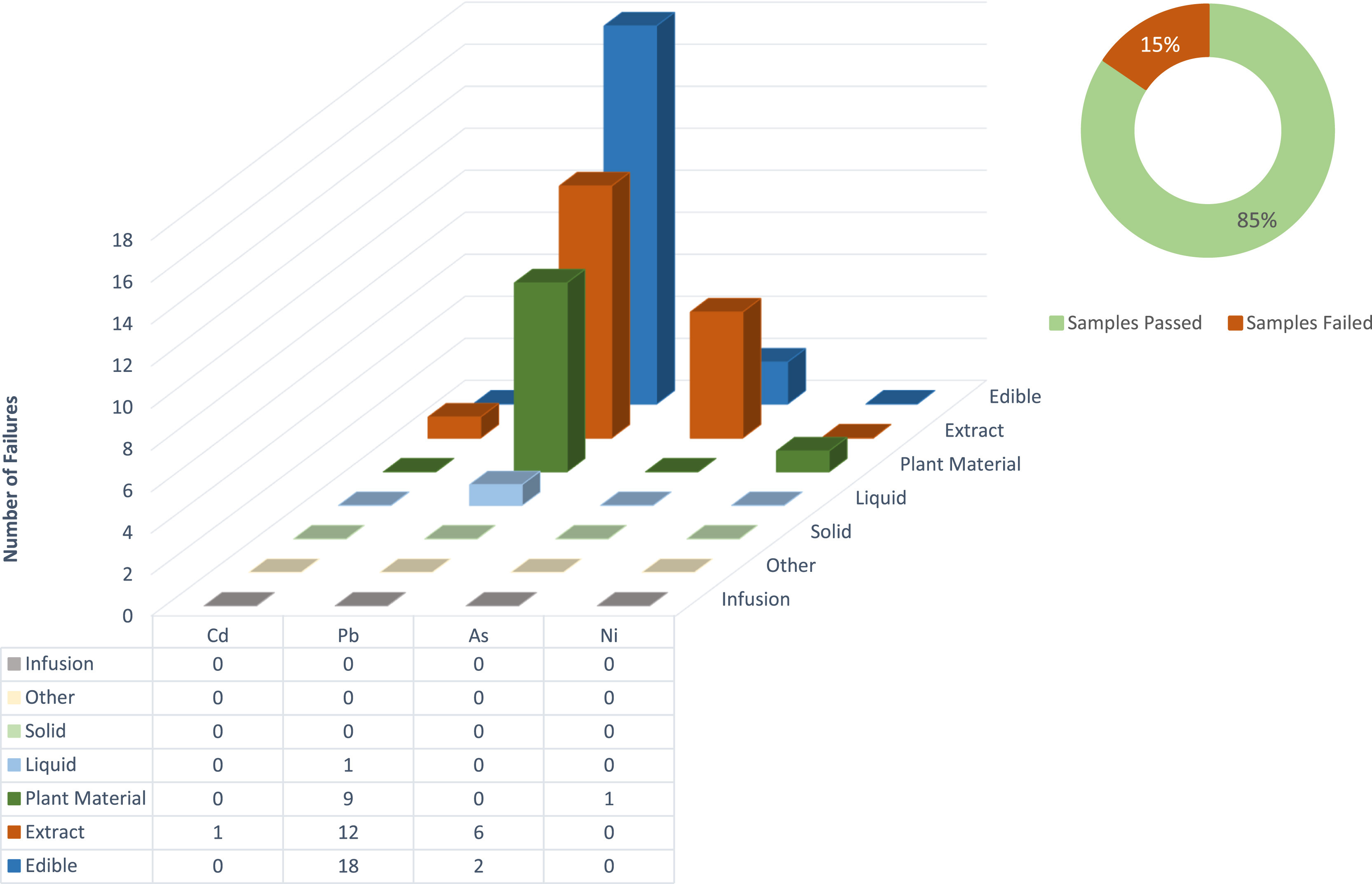
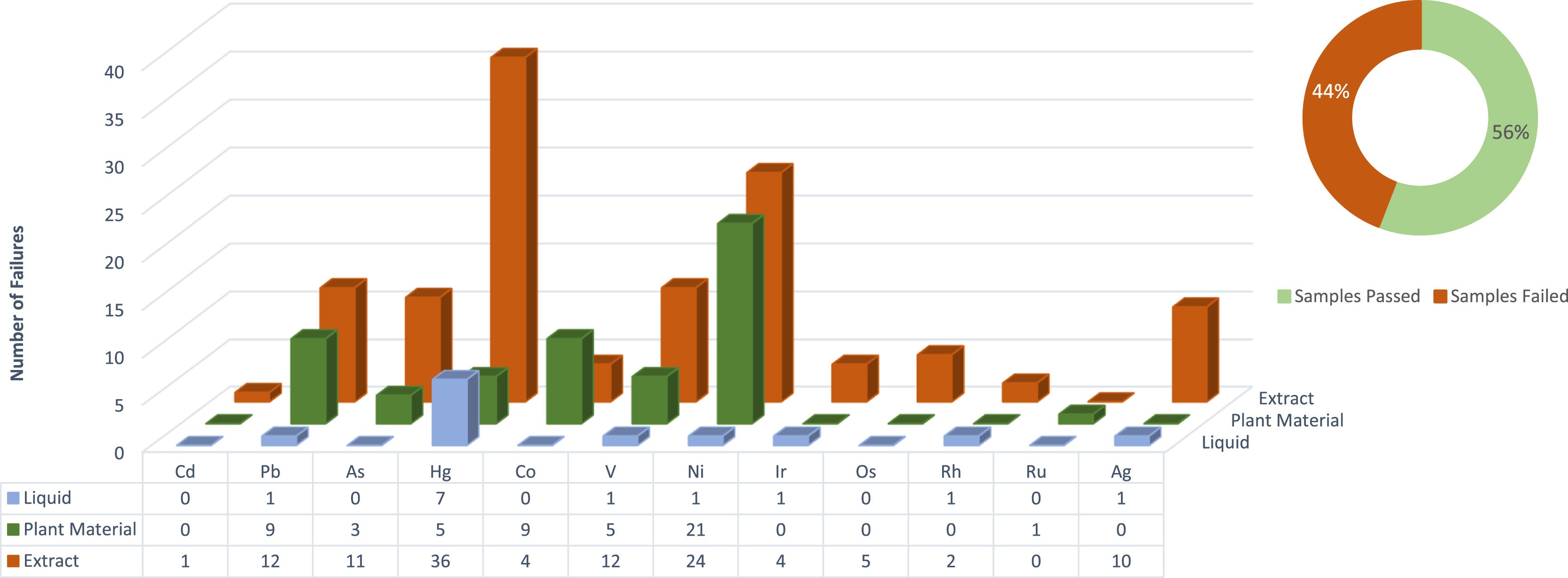
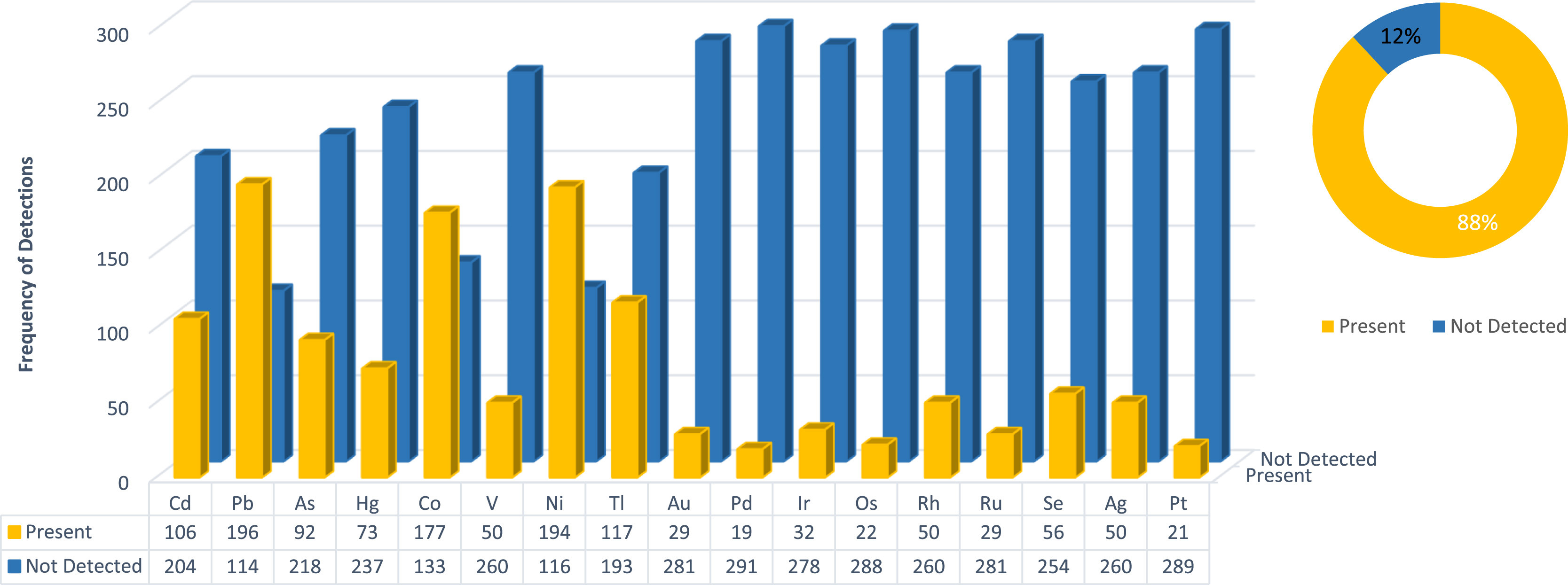
Tabulated results of the data are shown in Appendix A, Tables A1–A4. Individual elemental data are grouped within categories according to specification, as mentioned above. It should be noted that the applicable categories, together with metal residues that failed, will be displayed. If a metal was present in the test method but had no failures, it will not be displayed on the figures. The presence of each residue is also displayed for the analyzed 310 samples, together with the limit of detection for each residue (Appendix A, Table A3). Additionally, Appendix A, Table A4 shows the number of samples that failed when they were evaluated against the different specification limits. Furthermore, the table also shows the number of samples for which heavy metal residues could be detected, irrespective of the concentration. A visual representation of the data is given in Figures 1–3.
|
|
|
Discussion
Represented in this data set is a small portion of samples obtained from the South African market. It is by no means a representative sample of the entire South African market, but inferences can be made nonetheless. The dataset will be discussed in two sections, relating to individual heavy metal residues, as well as relating to samples.
Individual heavy metal residues
When comparing the individual heavy metal residues (Fig. 1) against the oral limit, it is evident that lead (Pb), arsenic (As), and nickel (Ni) are the metals most responsible for failures. Detection of Class 1 residues above the USP <232>/<233> and ICH Q3D oral specification limits were responsible for 91% (49 out of 54) of all residue failures identified in this study. It is further interesting to note that only four of the seven categories contained heavy metals at concentrations high enough to cause a failure. The categories that did not contain residues at concentrations high enough to fail could be a result of the concentration dilution being performed or, alternatively, the process removes metal residues to some degree. For example, when infusions are considered, a dilution of concentration cannabinoids is prepared, and consequently other residues like heavy metals and solvents are also diluted. As for solids, the process of manufacturing a pure CBD isolate usually entails a distillation or crystallization of sorts, which would remove metal residues to an extent.
When considering the more stringent inhalation USP <232>/<233> and ICH Q3D limits (Fig. 2), the three metals that were responsible for most failures were mercury (Hg), nickel (Ni), and lead (Pb). Furthermore, only three of the categories were considered for the inhalation limits since products from the other categories had none or low potential for being dosed in an inhalation form. It is evident that a clear increase in the number of residue failures is present when applying the more stringent inhalation limit. A 346% increase is observed in the number of failures detected when inhalation limit residues are compared to oral limit residues. Note that 46% (85 out of 187) of all failures are attributed to Class 1 residues, which is lower than the samples submitted for comparison with the oral limit. It should, however, be made clear that 87% (162 out of 187) of failures are attributed to a combination of Class 1 and Class 2A. Next to Class 1, Class 2A residues are the second most dangerous to human health. For samples submitted to be tested against the inhalation specification limit, a significant number were vape pens or vape pen attachments containing oil. These pens or attachments were manufactured with a transparent glass exterior to be able to view the level of oil, which is contained in a metal housing. Observations made suggest that the metal housing showed characteristics of nickel plating. This could explain the vast number of nickel failures detected, since nickel is most probably leached into the product after production in finished form. The small quantity of oil combined with the large surface area of the metal housing could leach enough nickel into products to cause detrimental health effects when inhaled.
If no limit is enforced and only occurrence or presence of a residue in a sample is considered (Fig. 3), four main residues make up 52% (684 out of 1313) of all residue occurrences. These residues include lead (Pb), cobalt (Co), nickel (Ni), and thallium (Tl). What is more concerning is that a Class 1 residue was detected in 36% (467 out of 1313) of the samples. This means that, among all residues analyzed, a Class 1 residue will be present in a third of all samples. Limits of detection are shown in Appendix A, Table A3 and Table A5.
Sample heavy metal residues
Considering the sample failures presented in Fig. 1's pie chart, a total 15% (48 out of 310) of all samples submitted for heavy metal residue analysis failed when compared against the USP <232>/<233> and ICH Q3D oral specification limit. These samples included a wide variety of product types, including drops, capsules, drinks, edibles, and suppositories. The latter was also considered under the oral specification limit although they are not exposed to low pH stomach acids, which could decarboxylate certain cannabinoids. A staggering increase in the number of failures for the ICH inhalation limit is evident compared to the oral limit presented in Fig. 2's pie chart. A total of 44% (94 out of 213) of all samples, in the applicable categories, submitted for heavy metal residue analysis failed when compared to the inhalation limit. This is a concern since almost half of all samples grouped in these categories failed. Sample types in this category included vape pens, raw plant material, and vape oils. Furthermore, when considering the individual residues, failures are attributed mostly to Class 1 residues when applying the oral limit, and Class 1 as well as Class 2A residues when comparing to the inhalation limit. Class 1 and Class 2A heavy metal residues are considered to be most detrimental to human health. Some 88% (273 out of 310) of samples contained heavy metal residues, irrespective of whether they were above or below specification limits, as seen in Fig. 3's pie chart, this data may be used to focus on the metal residues most predominantly found in the analyzed matrices.
Conclusion
When considering the results obtained, it is exceedingly necessary to have an appropriate quality control system in place, especially for heavy metal residues analysis in the current South African market. What is most worrying is the high failure rate in conjunction with the presence of mostly Class 1 and Class 2A residues. Even though published standards and safety limits exist, the enforcement of these limits needs to be taken much more seriously. This data should inform the public who purchase these products, as well as regulators and manufacturers, of the potential health endangerment of individuals who consume these products on a daily basis.
Appendix A
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Acknowledgements
National Analytical Forensic Services (NAFS) Laboratory. Laboratory Staff: Paul Myburgh, Jeanette Leygonie, Alexander Wrbka, Gerdus Kemp (CEO).
Author contributions
Hendrik Jacobus Viviers: Conceptualization, Methodology, Investigation, Data curation, Writing – original draft. Anél Petzer: Supervision, Writing – review & editing. Richard Gordon: Supervision, Writing – review & editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Author H.J. Viviers is employed at National Analytical Forensic Services (NAFS).
References
- ↑ 1.0 1.1 World Health Organization, ed. (2007). WHO guidelines for assessing quality of herbal medicines with reference to contaminants and residues. Geneva: World Health Organization. ISBN 978-92-4-159444-8. OCLC 232540335. https://www.worldcat.org/title/mediawiki/oclc/232540335.
- ↑ 2.0 2.1 Edelstein, Menahem; Ben-Hur, Meni (1 April 2018). "Heavy metals and metalloids: Sources, risks and strategies to reduce their accumulation in horticultural crops" (in en). Scientia Horticulturae 234: 431–444. doi:10.1016/j.scienta.2017.12.039. https://linkinghub.elsevier.com/retrieve/pii/S0304423817307628.
- ↑ Khan, Zafar Javed; Khan, Naeem Ahmad; Naseem, Imrana; Nami, Shahab A A (21 November 2017). "DETERMINATION OF HEAVY METALS, AFLATOXINS, MICROBIAL LOADS AND PESTICIDES RESIDUE IN SEHJANA (MORINGA OLEIFERA LAM) FRUITS/PODS". International Research Journal of Pharmacy 8 (10): 203–207. doi:10.7897/2230-8407.0810208. ISSN 2230-8407. https://doi.org/10.7897/2230-8407.0810208.
- ↑ Tchounwou, Paul B.; Yedjou, Clement G.; Patlolla, Anita K.; Sutton, Dwayne J. (2012), "Heavy Metal Toxicity and the Environment" (in en), Experientia Supplementum (Basel: Springer Basel): 133–164, doi:10.1007/978-3-7643-8340-4_6, PMC PMC4144270, PMID 22945569, https://doi.org/10.1007/978-3-7643-8340-4_6. Retrieved 2021-09-28
- ↑ Meers, E.; Ruttens, A.; Hopgood, M.; Lesage, E.; Tack, F.M.G. (1 October 2005). "Potential of Brassic rapa, Cannabis sativa, Helianthus annuus and Zea mays for phytoextraction of heavy metals from calcareous dredged sediment derived soils" (in en). Chemosphere 61 (4): 561–572. doi:10.1016/j.chemosphere.2005.02.026. https://linkinghub.elsevier.com/retrieve/pii/S0045653505002808.
- ↑ Marques, Ana P. G. C.; Rangel, António O. S. S.; Castro, Paula M. L. (10 August 2009). "Remediation of Heavy Metal Contaminated Soils: Phytoremediation as a Potentially Promising Clean-Up Technology". Critical Reviews in Environmental Science and Technology 39 (8): 622–654. doi:10.1080/10643380701798272. ISSN 1064-3389. https://doi.org/10.1080/10643380701798272.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 "Elemental Impurities Updates". United States Pharmacopeia. The United States Pharmacopeial Convention. 8 February 2016. https://www.usp.org/chemical-medicines/elemental-impurities-updates.
- ↑ 8.0 8.1 8.2 8.3 8.4 "ICH Q3D Elemental impurities". European Medicines Agency. 29 January 2021. https://www.ema.europa.eu/en/ich-q3d-elemental-impurities.
- ↑ 9.0 9.1 Fischer, Lisa; Zipfel, Barbara; Koellensperger, Gunda; Kovac, Jessica; Bilz, Susanne; Kunkel, Andrea; Venzago, Cornel; Hann, Stephan (1 July 2014). "Flow injection combined with ICP-MS for accurate high throughput analysis of elemental impurities in pharmaceutical products according to USP /" (in en). Journal of Pharmaceutical and Biomedical Analysis 95: 121–129. doi:10.1016/j.jpba.2014.02.016. https://linkinghub.elsevier.com/retrieve/pii/S0731708514001071.
- ↑ Elder, D.; Teasdale, A. (2014). "ICH Q3D (residual metals): Challenges and opportunities". Regulatory Rapporteur 11.
- ↑ Viviers, Hendrik Jacobus; Petzer, Anél; Gordon, Richard (1 May 2021). "An assessment of the potency related to Cannabis-based products in the South African market" (in en). Forensic Science International 322: 110754. doi:10.1016/j.forsciint.2021.110754. https://linkinghub.elsevier.com/retrieve/pii/S0379073821000748.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. References aren't listed in the order they appear in the original, but they do for this version, by unavoidable design. In some cases important information was missing from the references, and that information was added. A few words were added, updated, or shifted for improved grammar and readability, but this version is otherwise unchanged in compliance with the "NoDerivatives" portion of the original's license.