Informatics Educational Institutions & Programs
Contents
| |
| Names | |
|---|---|
| IUPAC name
Europium(II) iodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.040.641 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| EuI 2 | |
| Molar mass | 405.77 g/mol |
| Appearance | Tea yellow powder |
| Melting point | 510 °C (950 °F; 783 K) |
| Boiling point | 1,120 °C (2,050 °F; 1,390 K) |
| Soluble in THF | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Europium(II) iodide is the iodide salt of divalent europium cation.
Preparation
Europium(II) iodide can be prepared in a handful of ways, including:
Reduction of europium(III) iodide with hydrogen gas at 350 °C:[1]
- 2 EuI3 + H2 → 2 EuI2 + 2 HI
Thermal decomposition of europium(III) iodide at 200 °C:[1]
- 2 EuI3 → 2 EuI2 + 2 I2
Reaction of europium with mercury(II) iodide:[1]
- Eu + HgI2 → Eu I2 + Hg
Reaction of europium with ammonium iodide:[1]
- Eu + 2 NH4I → EuI2 + 2 NH3 + H2
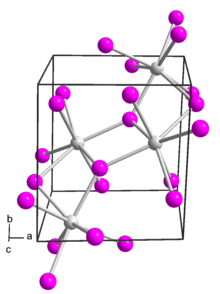
Structure
Europium(II) iodide has several polymorphs.[2] It adopts a monoclinic crystal structure in space group P 21/c (no. 14).[3][4]
It also adopts an orthorhombic polymorph in space group Pbca (no. 61). This form is isostructural with strontium iodide.[5]
A third polymorph of europium(II) iodide is formed if it is prepared from europium and ammonium iodide at low temperatures (200 K) in liquid ammonia. This low-temperature phase is orthorhombic and in space group Pnma (no. 62). This is the same structure as modification IV of strontium iodide.[6]
References
- ^ a b c d Brauer, Georg (1975). Handbook of Preparative Inorganic Chemistry. ISBN 3-432-02328-6.
- ^ Wells, A. F. (1984). Structural Inorganic Chemistry (5th ed.). Oxford University Press. pp. 415–416. ISBN 978-0-19-965763-6.
- ^ Bärnighausen, H.; Beck, H.; Grueninger, H. W.; Rietschel, E. T.; Schultz, N. (1969). "Neue AB2-strukturtypen mit siebenfach koordiniertem kation". Z. Kristallogr. 128: 430.
- ^ Bärnighausen, H.; Schulz, N. (1969). "Die Kristallstruktur der monoklinen Form von Europium(II)-jodid EuJ2". Acta Crystallogr. B. 25: 1104–1110. doi:10.1107/S0567740869003591.
- ^ Bärnighausen, H.; Beck, H. P.; Grueninger, H. W. (1971). "Search Results - Access Structures". Rare Earths Mod. Sci. Technol. 9: 74–.
- ^ Krings, M.; Wessel, M.; Dronskowski, R. (2009). "EuI2, a low-temperature europium(II) iodide phase". Acta Crystallogr. C. 65: i66–i68. doi:10.1107/S0108270109038542.

















