Pharmaceutical Manufacturing Flows – Making the Complex Easy
The secret to successful LIMS implementations are understanding the pinch points and what information is important. This is never more true than in pharmaceutical manufacturing flows, where clear requirements can capture the flow and distill the complexity to craft a robust solution.
Features of a Manufacturing Flow
All manufacturing flows, whether making cars, pies, medical devices, or pharmaceuticals, take raw materials, and through a series of manufacturing processes, transform them into a final product. In pharmaceutical production the constituent parts include the active pharmaceutical ingredient (API), excipients, and packaging and labelling materials. Pharmaceuticals in tablet form may have coating agents added to make them easier to swallow and coloring to differentiate them. Tablets are often packaged in blister packs of 20 or so with a cardboard carton on which key consumer information is printed. As with cars or ice-cream each constituent part from the API to the foil of the blister pack must be traceable back to the originating batch of each originating supplier. If multiple batches of the same material are used this must become part of the production record. The key is traceability; traceability of everything that went into the batch of a product.
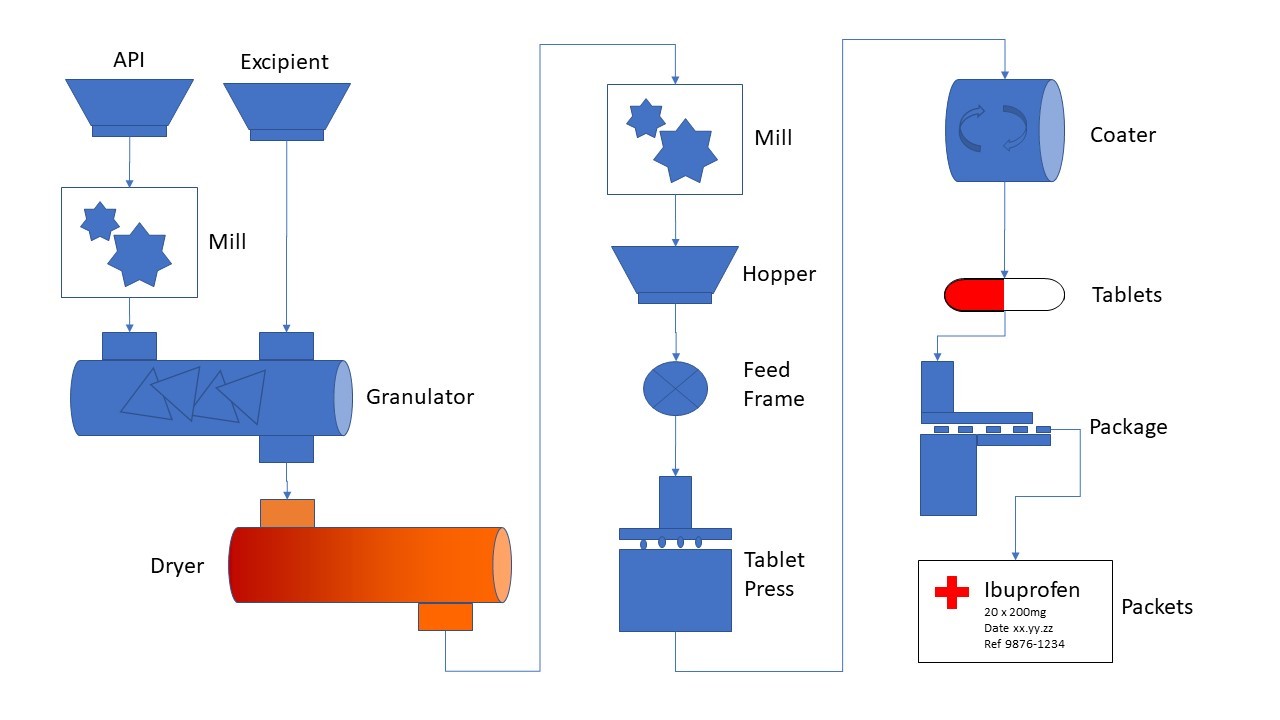
Figure 1: Typical pharmaceutical tablet production line
An overview of a typical pharmaceutical tablet production line is shown in Figure 1. Like cooking, the exact proportions of the various raw ingredients are controlled by a recipe which defines the amount of API, and excipient required. The various ingredients may be milled and mixed to ensure an even distribution of API. Mixing can be performed as a slurry/wet mix which is dried and milled again to create a fine powder. This bulk powder may be mixed with further excipients before being formed into tablets in a tableting machine. The tablets may be coated and finally packaged as required.
Testing will be performed at each critical stage of the flow from raw materials to the final packaged product. For example, the incoming raw materials may be re-tested to ensure they conform to the supplier’s specification before being released for use. The output of the granulator may be tested to ensure the API is evenly mixed and the output from the milling checked to ensure the mixture still contains the correct proportions of API and is ground evenly. Samples of tablet from the final product are retained for dissolution, stability, and other quality assurance purposes.
Requirements of a Pharmaceutical Manufacturing LIMS
All LIMS require the ability to track samples and associated test results, create certificates of analysis (CofA), use barcoding and associate samples with key information such as batch and supplier. LIMS will also include the ability to manage calibration and maintenance of laboratory instruments, and records which instruments were used for each sample test, along with who tested that sample and whether they were competent to test it, and to control inventory of the chemicals and the laboratory consumables used in testing.
But while a typical QC sampling flow monitors and reports on discreet samples, a manufacturing flow requires a broader range of information to be associated and combined for management and reporting. A manufacturing flow requires the addition of Recipe Management to control the raw materials and intermediate products going into each manufacturing batch; the ability to keep test results for each part of the flow (raw materials, intermediate stages and final product); to link test results to the originating raw materials/suppliers and final product/customers; the statistical tools to control the process within defined limits; and the ability to quickly retrieve and report on this data.
Key parts of a pharmaceutical manufacturing LIMS include:
- Specification Management
- Manages the specification of the Raw Material (RM), Intermediate Product (IP) and Final Product (FP)
- Recipe Management
- Defines the components of the product
- Batch Management
- Manages RM, IP, and FP batches within the manufacturing flow
- Batch Genealogy
Defines the relationship between raw materials and final product to trace which batches are used in which products - Manufacturing Sample Management
Defines batch and sample templates (with associated tests) required for testing RM, IP, and FP - Material and Inventory Management
Manages the inventory of physical samples taken as part of the manufacturing process. Also manages inventory of laboratory consumables, reagents, and chemicals - Supplier Management
Manages suppliers of materials including reagents, consumables, and raw materials - Instrument Calibration and Maintenance
Manages instruments and equipment used within the laboratory ensuring only correctly calibrated and maintained instruments are used for testing - Competency and Training Management
Manages staff training records and the ability to restrict tasks and actions to competent staff - Method Management
Manages the methods associated with analytical tests - CAPA
Manages the quality improvement cycle through corrective actions/preventative actions - Skip Lot/Selective Testing
Manages reduced testing based on defined rules (i.e. test every 20 after 5 good tests) - Work Assignment
Assigns analysts and instruments to tests - Runsheet Management
Manages the creation of analytical runsheets that defines sample test order including blanks and duplicates - Instrument Integration
Integrates with analytical instruments to automate the transfer of results to the LIMS - External ERP Interface
Integrates with external enterprise resource planning solutions (such as SAP). Import of inspection plans and inspection lots and the export of the batch usage decision and analytical results - Product Grading
Manages the assignment of a grade to a product once it has been tested - Chain of Custody
Records a full chain of custody for a sample including both location and responsible person - Certificate of Analysis
Manages the generation of Certificates of Analysis (CoA) and Certificates of Conformance (CoC) - Advanced Analytics
Provides useful statistics about the product (conformance and variation), the laboratory (speed and efficiency), as well as suppliers, customers, and raw materials - Stability Testing
To determine the retention of product quality over time, for shelf-life purposes















