Simplifying Results Collection and Approval in Laboratories
Of all the functionality offered by modern LIMS, accurate acquisition and reporting of test results is a central requirement. There are essentially two ways to enter results data into the LIMS – manually or directly from the testing instruments. Entering results manually is clearly quite common, but offers the possibility for transcription errors, especially if the results are first written down in a notebook and then later typed into the LIMS. Direct integration of instrumentation into the LIMS eliminates this possibility and helps meet data integrity and validity requirements. This is particularly important in the pharmaceutical manufacturing industry, where data integrity is critical throughout the cGMP (Good Manufacturing Practice) data life cycle. Data integrity refers to the completeness, consistency, and accuracy of data. Complete, consistent, and accurate data should be attributable, legible, contemporaneously recorded, original or a true copy, and accurate (ALCOA).
Not only does direct entry of results data into Matrix Gemini eliminate transcription errors, it provides higher levels of automation, and improves laboratory efficiency. Many laboratories handle thousands of samples per year, with multiple tests being carried out on each sample. Entering all of these test results manually can be extremely time consuming, so direct transfer can lead to an enormous improvement in laboratory efficiency.
Automating Results Collection
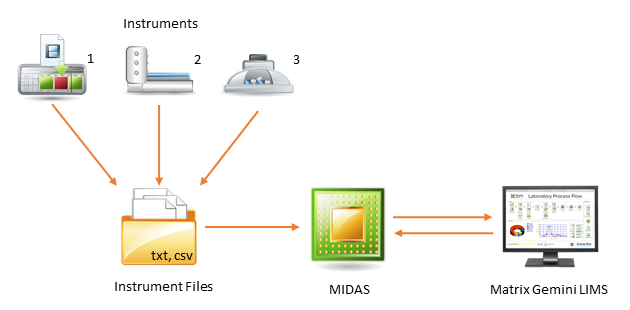
To help with the automation for results data collection, we have recently introduced the new MIDAS (Matrix Instrument Data Acquisition System) module which improves on the existing methods of interfacing of a wide variety of analytical instruments and equipment to Matrix Gemini, from PCR and ELISA instrumentation to chromatographs. Many laboratory instruments are able to output result files, usually in CSV or text format. Running in the background, MIDAS monitors the creation of these files and imports results directly into Matrix Gemini when a new file is detected. This includes result files based on worksheets created by the Matrix Gemini worksheet functionality. Relevant data is extracted from the instrument file and associated with the correct data in Matrix Gemini LIMS. Results are automatically checked against relevant specification limits as the data is imported.
By recording measurement data at the point that it is generated, MIDAS also simplifies the results review and approval process. Any need to manipulate data in third party packages such as Excel is eliminated. Matrix Gemini provides status driven validation and approval stages which can be configured according to the requirements of any particular laboratory. Approval allows the test results for individual samples to be selected for review. The user can then assign an ‘Approved’ or ‘Rejected’ status to the samples. However, it is also possible to configure the system for automatic approval if all of a sample’s test results fall within the test limits associated with it. Since completion of approval generally triggers the final release of the results to the person who submitted the samples (unless some sort of invoicing is also initiated by the approval step), this marks the completion of the sample workflow. Thus using direct test result entry in combination with automated approval procedures even further improves laboratory efficiency. Not only that, the MIDAS module allows users to instantly setup or modify instrument interfaces themselves avoiding the time delays and costs traditionally involved in linking and upgrading instruments.















