August 18, 2020 - Importing Spreadsheets and CSV Files into LabVantage LIMS
Two of the most popular file formats used in science settings are Excel spreadsheets and CSV (Comma Separated Value) files. So, it comes as no surprise to hear that they are heavily relied on in the laboratory informatics market as well. Teams using a LIMS frequently collaborate on analyzing test result data, which often involves columns and tables. Formulas to analyze the data can be added via most spreadsheet editing programs, and these calculations may be crucial to seeing patterns in the data.[Read More]
August 18, 2020 - Designing the Unified Lab of the Future with BIOVIA ONE Lab
 The need for optimizing operational efficiency while maintaining data integrity is at the core of every laboratory informatics roadmap. Innovative technologies such as BIOVIA ONE Lab, that enable digital transformation holds the key to driving process improvement and automating daily tasks within the laboratory, but the challenge lies within how to go about this effort?
The need for optimizing operational efficiency while maintaining data integrity is at the core of every laboratory informatics roadmap. Innovative technologies such as BIOVIA ONE Lab, that enable digital transformation holds the key to driving process improvement and automating daily tasks within the laboratory, but the challenge lies within how to go about this effort?
[Read More]
August 11, 2020 - National Institutes of Health (NIH) Research Updates – August 2020
The National Institutes of Health (NIH) is our nation’s medical research agency and strives to make scientific discoveries that improve health and save lives. Founded in 1870, the NIH conducts its own scientific research through its Intramural Research Program (IRP), which supports approximately 1,200 principal investigators and more than 4,000 postdoctoral fellows conducting basic, translational and clinical research. In this blog, we will highlight recent ground-breaking NIH research.[Read More]
August 11, 2020 - Guide to Laboratory Information Management System Software Validations
 Clinical laboratories are often uncertain about the requirements for validation across the Laboratory Information Management System (LIMS) lifecycle. After your laboratory adopts a new LIMS, you must perform a full LIMS validation prior to using...
Clinical laboratories are often uncertain about the requirements for validation across the Laboratory Information Management System (LIMS) lifecycle. After your laboratory adopts a new LIMS, you must perform a full LIMS validation prior to using...
[Read More]
August 11, 2020 - LIMS Data Analytics and Visualization
LIMS Data Analytics and Visualization tools graphically present data from your LIMS, and potentially other applications, to provide powerful business intelligence for your laboratory and organization. Like all LIMS, Matrix Gemini uses a relational database (usually Microsoft SQL or Oracle) to store the LIMS data.[Read More]
August 11, 2020 - Sunquest Physician Portal SM for Sunquest Mitogen ™ LIMS
[LEARN MORE]
August 4, 2020 - Start or Scale Molecular Testing with Sunquest Mitogen™ LIMS
 Sunquest Mitogen LIMS is a cloud-based, scalable, purpose-built solution for executing on molecular and genetic testing. Not only does our offering contain the critical components of a fully-featured molecular LIMS, but also it includes the patient accessioning and reporting features normally seen in a clinical laboratory information system (LIS).
Sunquest Mitogen LIMS is a cloud-based, scalable, purpose-built solution for executing on molecular and genetic testing. Not only does our offering contain the critical components of a fully-featured molecular LIMS, but also it includes the patient accessioning and reporting features normally seen in a clinical laboratory information system (LIS).
[READ MORE]
August 3, 2020 - Coronavirus Treatments That Work

The current official stance is that there is no real treatment for COVID-19. If you get it, you are told to just go home and tough it out…until/unless you start finding it hard to breathe, or develop other severe symptoms requiring hospitalization. Fortunately, in the vast majority of cases (something like 80%) symptoms are mild, moderate or non-existent. And, moreover, the mortality rate...
[Read More]
July 29, 2020 - Taking Advantage of LabVantage LIMS Workflows
 LabVantage Workflow Designer is a LabVantage LIMS version 8 module that allows users to divide a complex laboratory process into manageable units of work to drive automation throughout the system. The module consists of functionality that guides users through the execution of predefined operations called Tasks. Samples and other laboratory data flow through the workflow,…
LabVantage Workflow Designer is a LabVantage LIMS version 8 module that allows users to divide a complex laboratory process into manageable units of work to drive automation throughout the system. The module consists of functionality that guides users through the execution of predefined operations called Tasks. Samples and other laboratory data flow through the workflow,…
[Read More]
July 29, 2020 - Benchling Case Study Anthology: Pushing the Frontier of Life Science Innovation
From next-generation cell therapies, to CRISPR-based gene therapies, to biologically-derived materials, life science R&D has never been more complex or more varied. But despite the explosion in scientific techniques and applications, modern life science companies have one challenge in common: taming the complexity of their data. Paper notebooks, spreadsheets, and legacy software like ELNs and LIMS can’t handle the complexity of modern life science.[Read More]
July 29, 2020 - Pharmaceutical Manufacturing Flows – Making the Complex Easy
The secret to successful LIMS implementations are understanding the pinch points and what information is important. This is never more true than in pharmaceutical manufacturing flows, where clear requirements can capture the flow and distill the complexity to craft a robust solution.July 29, 2020 - The Sunquest COVID-19 Essential Suite
 Extend your reach to physician customers and test for both active and past infections.
Extend your reach to physician customers and test for both active and past infections.
The Sunquest COVID-19 Essential Suite provides independent and state labs with a set of tools to quickly stand up testing and broaden interoperability reach to physician customers. The offering includes rapid implementation of electronic COVID-19 test orders and reliable results support, instrument connectivity, and workflows for both active and past infection through RT-PCR and serology testing.
[Read More]
July 29, 2020 - A Better Tomorrow! Navigating the Next Normal
Amidst the Covid19 crisis, industries have a unique opportunity to pause and reassess their future and long-term strategy. The Pharma Industry has the dual challenge of securing its feet on the ground and at the same time, saving the world! In this situation, are Pharma companies prepared to navigate the next normal successfully?July 14, 2020 - Interfacing Your SampleManager LIMS™ to SAP QM
 Have you been thinking about integrating your SampleManager LIMS™ with your SAP system? Does it seem like interfacing is too complicated? Unsure if there’s enough benefit to justify the cost? You’re not alone. Some people question if a LIMS is necessary at all, given the release of SAP’s S/4HANA and enhancements to SAP QM. We wrote about some answers to that age-old question in a previous blog.
Have you been thinking about integrating your SampleManager LIMS™ with your SAP system? Does it seem like interfacing is too complicated? Unsure if there’s enough benefit to justify the cost? You’re not alone. Some people question if a LIMS is necessary at all, given the release of SAP’s S/4HANA and enhancements to SAP QM. We wrote about some answers to that age-old question in a previous blog.
[Read More]
July 14, 2020 - National Institutes of Health (NIH) Research Updates – July 2020
 The National Institutes of Health (NIH) is our nation’s medical research agency and strives to make scientific discoveries that improve health and save lives. Founded in 1870, the NIH conducts its own scientific research through its Intramural Research Program (IRP), which supports approximately 1,200 principal investigators and more than 4,000 postdoctoral fellows conducting basic, translational and clinical research. In this blog, we will highlight recent ground-breaking NIH research.
The National Institutes of Health (NIH) is our nation’s medical research agency and strives to make scientific discoveries that improve health and save lives. Founded in 1870, the NIH conducts its own scientific research through its Intramural Research Program (IRP), which supports approximately 1,200 principal investigators and more than 4,000 postdoctoral fellows conducting basic, translational and clinical research. In this blog, we will highlight recent ground-breaking NIH research.
[Read More]
July 14, 2020 - Automate Your Genetics Lab Testing with the Most Trusted and Widely Used Name in Genetics Laboratory Information Systems
SCC Soft Computer’s SoftGenomics® supports ordering, testing, documentation, and reporting in laboratories that perform testing using cytogenetic and molecular technologies, including chromosome analysis and microarrays, with instrument and foreign system interfacing capabilities and Web-based online ordering, reporting, and collaboration functionality. Regardless of your facility’s size or type of testing you perform, with built-in instrument connectivity and workflow automation, SCC’s Genetics Information Systems Solutions are designed to optimize your laboratory performance.[Read More]
July 7, 2020 - SCC Soft Computer Introduces 90-Day Rapid Implementation Featuring New Product SoftLIMS™
As laboratories prepare for an influx of testing due to COVID-19, SCC has established a rapid implementation of 90-days or less to onboard and accelerate COVID-19 tests. SCC’s Rapid Implementation process gets laboratories up and running within 90-days in order to support the rapid growth of current testing within the healthcare marketplace. Starting with identifying the client’s goals, such as to minimize manual data entry/electronic documentation or to increase productivity via automation, the idea for many is to streamline the current processes to accelerate resulting as testing increases exponentially as months go on.[Read More]
July 7, 2020 - What Is IQ OQ PQ in Software Validation
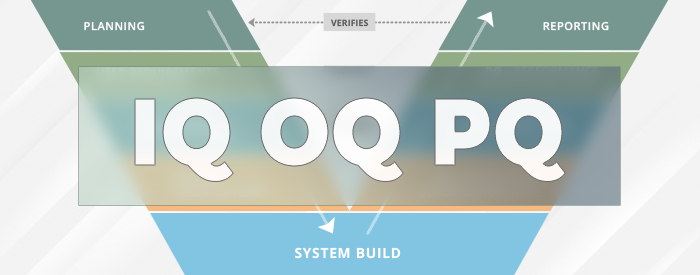 If you work in a business regulated by a government agency, once you’ve implemented or upgraded your LIMS, ELN, or CDS, you need to validate it for your intended use. One part of the validation process that often seems confusing to our clients is the IQ, OQ, PQ testing. This blog will demystify these validation protocol test scripts.
If you work in a business regulated by a government agency, once you’ve implemented or upgraded your LIMS, ELN, or CDS, you need to validate it for your intended use. One part of the validation process that often seems confusing to our clients is the IQ, OQ, PQ testing. This blog will demystify these validation protocol test scripts.
[Read More]
July 7, 2020 - Case Study: Cybersecurity Assessment for a Global Biopharmaceutical Company
 One of the world’s leading biopharmaceutical companies, initiated an Enterprise Cyber Resiliency initiative to protect and isolate its manufacturing sites from cyber-attack and have the ability to sustain operations in light of a corporate-level attack. Due to Astrix Technology Group’s (Astrix) expertise with enterprise-wide laboratory information systems, experience working with other biotech and pharmaceutical companies, and track record of eliminating risk and delivering on similar projects for other life science organizations, the customer chose Astrix to conduct an assessment of its laboratory systems across five global manufacturing sites.
One of the world’s leading biopharmaceutical companies, initiated an Enterprise Cyber Resiliency initiative to protect and isolate its manufacturing sites from cyber-attack and have the ability to sustain operations in light of a corporate-level attack. Due to Astrix Technology Group’s (Astrix) expertise with enterprise-wide laboratory information systems, experience working with other biotech and pharmaceutical companies, and track record of eliminating risk and delivering on similar projects for other life science organizations, the customer chose Astrix to conduct an assessment of its laboratory systems across five global manufacturing sites. [Read More]
June 29, 2020 - Customer Specification Matching and Product Regrading with QLIMS
 Product variation is a natural effect of manufacturing, the question here is how do you manage product variation in a manner that can improve your business and meet customer specifications.
Product variation is a natural effect of manufacturing, the question here is how do you manage product variation in a manner that can improve your business and meet customer specifications.
















