Posted on July 17, 2023
By LabLynx
Journal articles
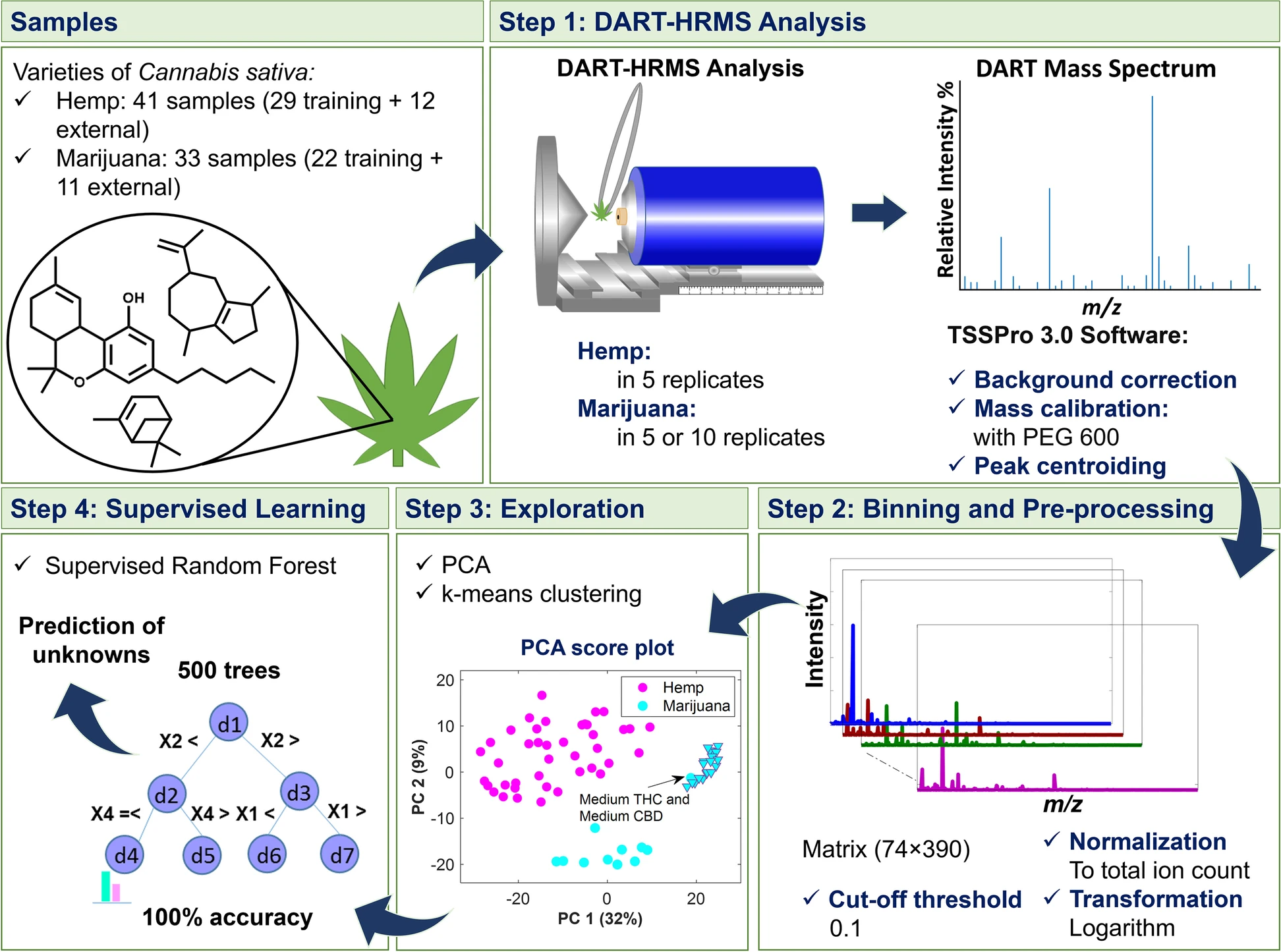
In this 2023 article published in the journal
Journal of Cannabis Research, Chambers
et al. of State University of New York present an analytical method for "the analysis and differentiation of
C. sativa plant materials" that, on paper, represent a major boost over more traditional chromatographic methods. After providing some background on those traditional types of analyses, the authors then present their method of using direct analysis in real time high-resolution mass spectrometry (DART-HRMS) with advanced chemometrics, including results associated with use of their method in a controlled laboratory environment. After some discussion, the authors conclude that their developed method "was successfully used to create a prediction model that facilitated rapid high-accuracy differentiation of
C. sativa hemp and marijuana plant materials obtained from multiple sources (i.e., commercial, DEA-registered, recreational)," while also noting that "100% accuracy in prediction was observed," making it an important potential future method "for the optimal differentiation of hemp and marijuana."
Posted on July 10, 2023
By LabLynx
Journal articles
Just as laboratories of all types managing complex workflows and large amounts of test data can benefit from laboratory information management systems (LIMS), similarly do clinical research groups conducting clinical trials benefit from software-based data and workflow management solutions, including the clinical trial management system (CTMS). From more standardized results and documents to more efficient workflows, such clinical systems are a boon to hospitals and research organizations looking to streamline process management for clinical trials. Highlighting this benefit, Shen
et al. present their custom CTMS for the First Affiliated Hospital at Zhejiang University School of Medicine (FAHZU) in this 2023 article published in
BMC Medical Informatics and Decision Making. After a thorough explanation of their system development and implementation, as well as discussion concerning its use, the authors conclude that the FAHZU CTMS "fully realizes the whole-process data management of clinical trials from project approval and review management to operational management," while providing "a variety of access methods to complete efficient data integration with [other] clinical business systems."
Posted on July 3, 2023
By LabLynx
Journal articles
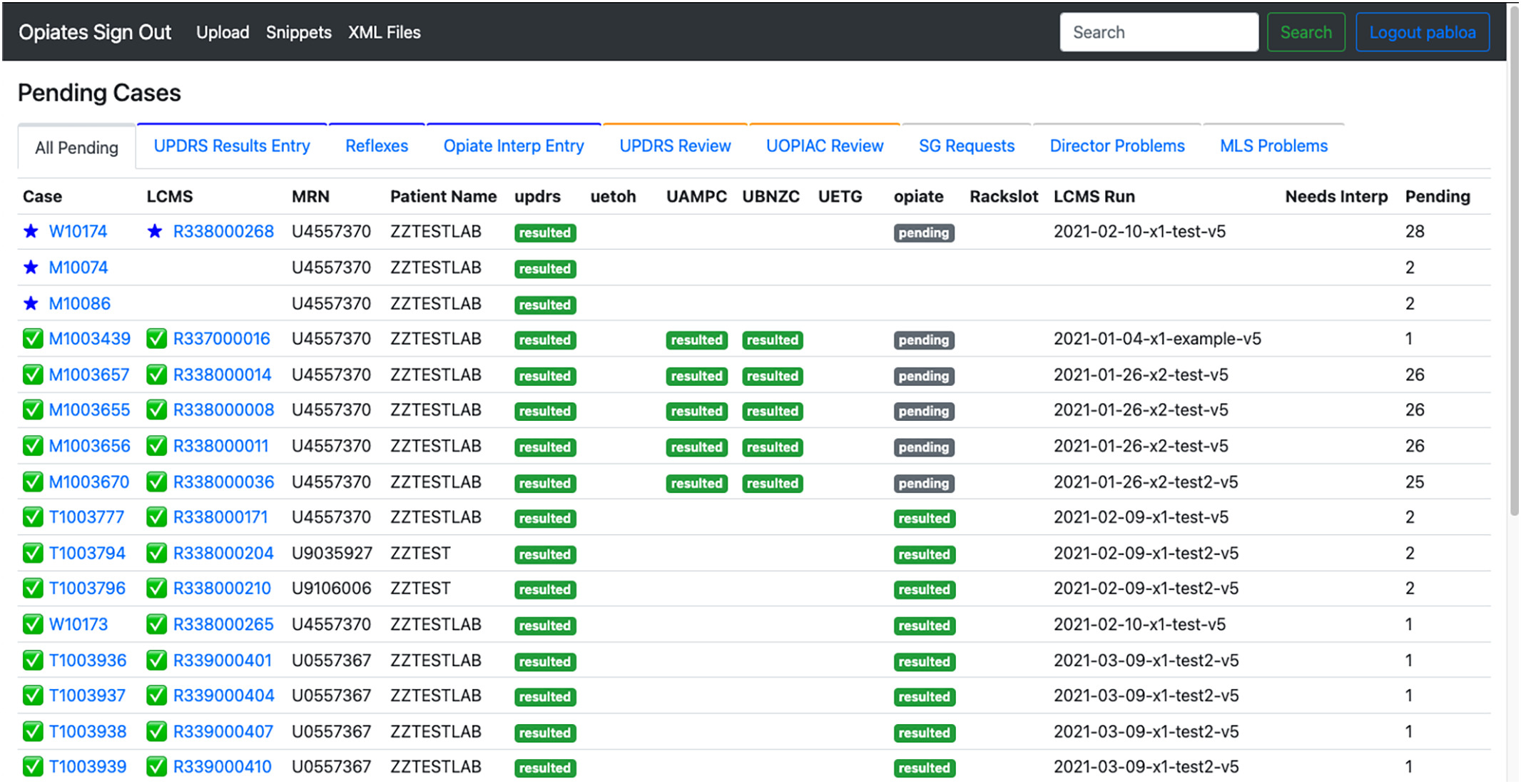
In this 2023 article published in the
Journal of Pathology Informatics, Pablo
et al. of the University of Washington School of Medicine present their work on developing a web-based application for better supporting the complex workflows of labs conducting pain-management-related toxicology and pathology testing. Noting a lack of support of these workflows among traditional laboratory information systems (LISs), the authors describe their approach to developing a system to meet their needs. After providing background and discussing the development approach, the authors present the results of their system implementation and performance analysis. After some discussion, the authors conclude that the "implementation of a purpose-built application to support reflex and interpretation workflows in a clinical pathology practice has led to a significant improvement in laboratory efficiency," adding that their custom, purpose-built application is able to "reduce staff burnout, reduce transcription errors, and allow staff to focus on more critical issues around quality."
Posted on June 26, 2023
By LabLynx
Journal articles
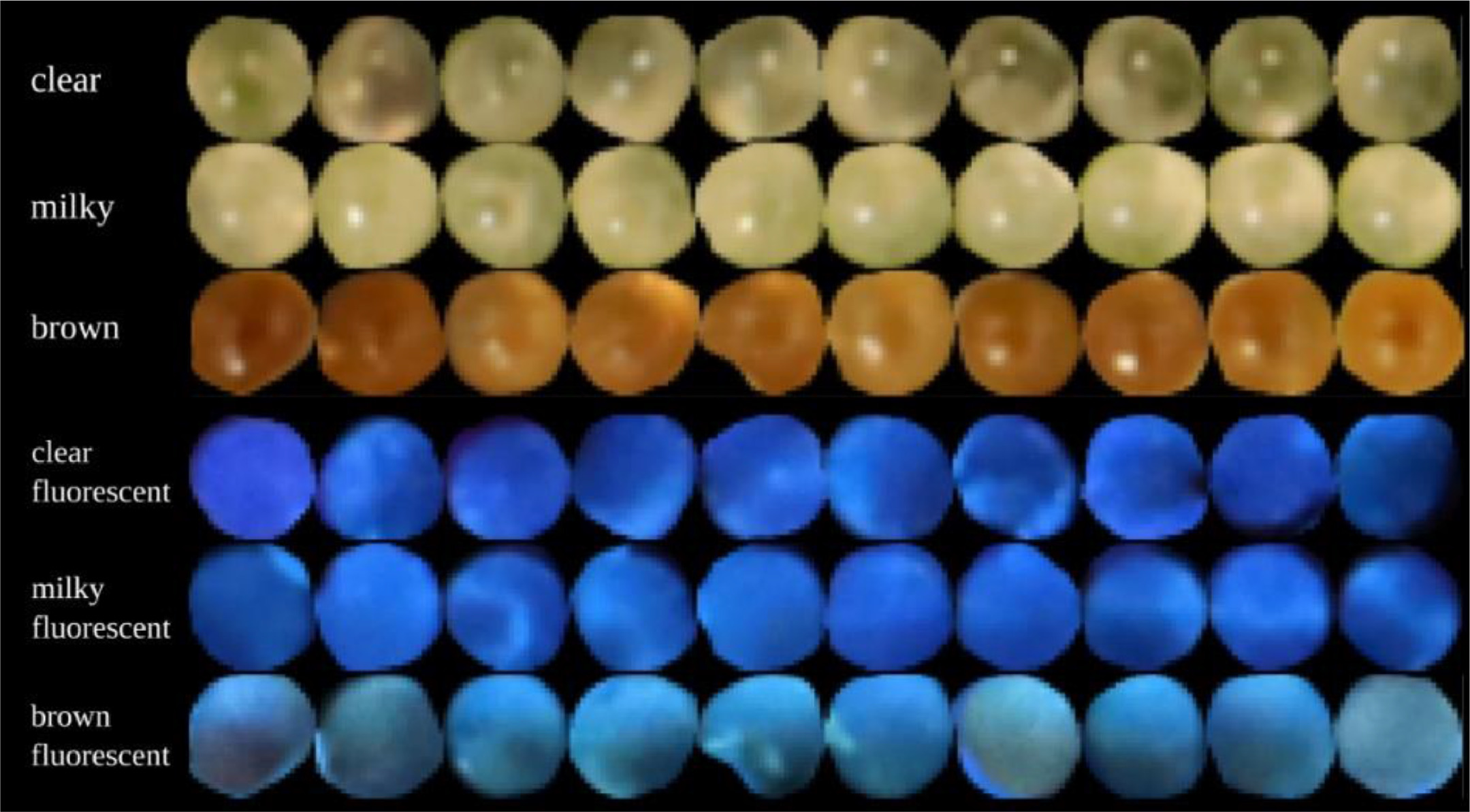
In this 2023 article published in the journal
Smart Agricultural Technology, Sutton
et al. propose an automated method for analyzing the trichomes of the
Cannabis inflorescences for their cultivation maturity. Noting "no scientifically based methods to predict maturation of inflorescences" in the literature, the authors sought out a computational method able to " extract trichome phenotype and morphology metrics during
Cannabis flower development from macroscopic photographs." After providing a brief introduction and a discussion of related work, the researchers discuss their materials, analytical methods, and data collection approach, followed by a lengthy explanation of their experimental results. The authors conclude that through their methods, "the observed relationship between trichome gland head diameter and morphological metrics indicates the feasibility of automatic quality assurance software that can determine how flower maturation is proceeding and if strain-specific potential may be attained." They add that further expansion on their work may produce real practical solutions for cultivators gauging the harvest-readiness of their
Cannabis plants.
Posted on June 20, 2023
By LabLynx
Journal articles
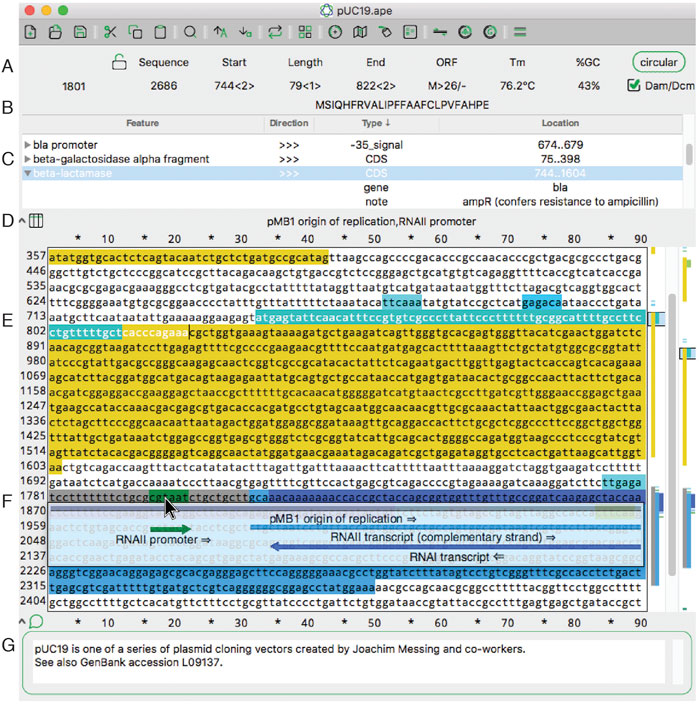
In this 2022 journal article published in
Frontiers in Bioinformatics, Davis and Jorgensen of the Howard Hughes Medical Institute and School of Biological Sciences at University of Utah discuss their free, multi-platform software for visualizing, designing, and presenting DNA materials and sequences. Noting that many such applications are out of reach of the small academic or teaching laboratory, the authors have developed and maintained A Plasmid Editor (ApE) for over 17 years, becoming a robust and versatile tool for molecular biologists. After introducing the software in the context of other such software, both free and commercial, they discuss their approach to developing the software over the years, followed by an extensive review of its features and functions. The authors conclude that ApE continues to be a solid " platform for creating visually appealing linear and circular plasmid maps," as well as simulate molecular techniques, adding "that many of its features and user interfaces have been inspired by input from users requesting new or modified functionality" over the years.
Posted on June 12, 2023
By LabLynx
Journal articles
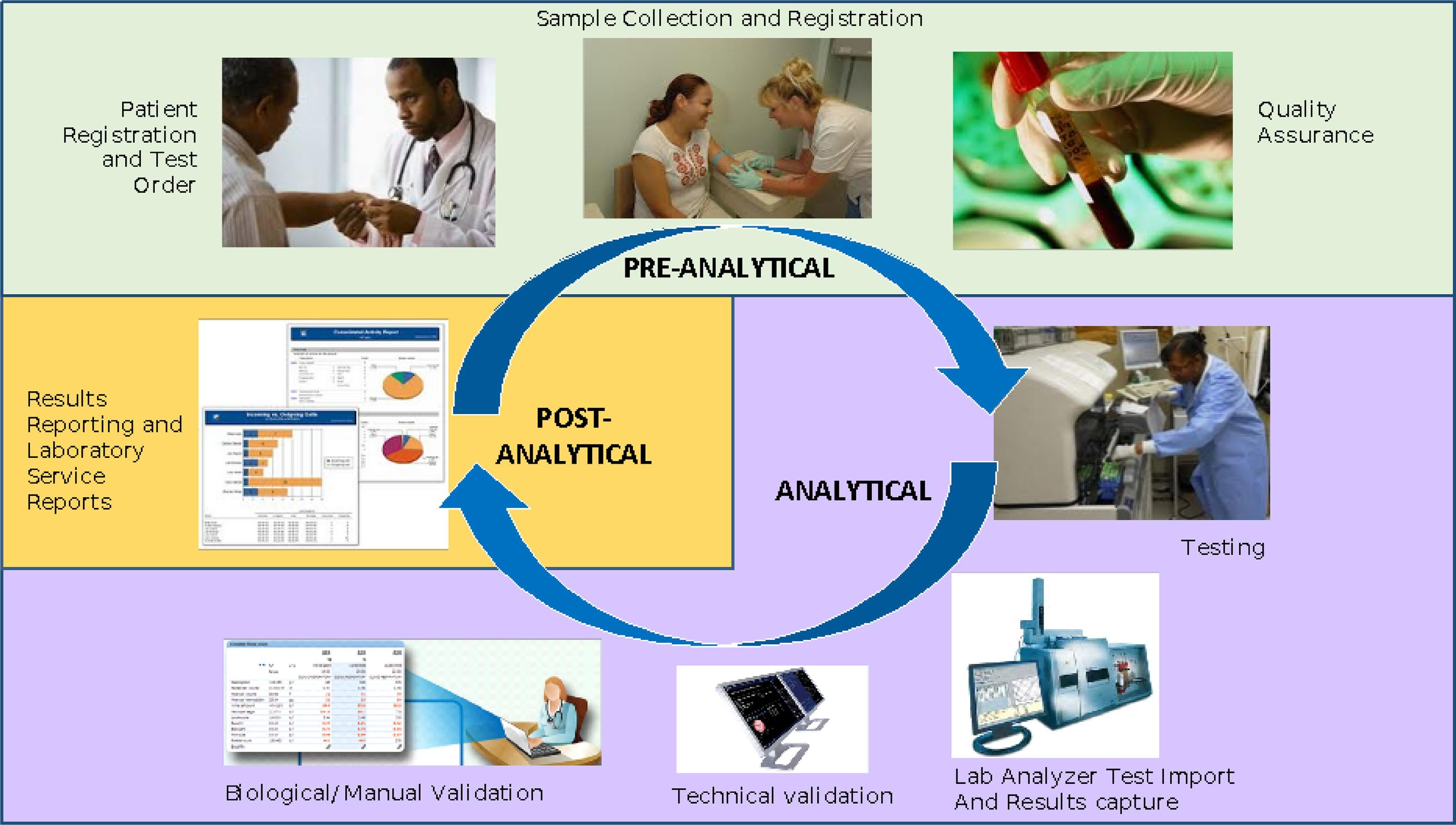
OpenELIS, a free open-source laboratory information system (LIS) for public health laboratories, has been around since 2012 and continue to garner new adopters. But what of the software more than a decade later? Is it still relevant and updated, and has the software demonstrated elements of sustainability for those who have installed it? In this 2023 paper published in the
International Journal of Medical Informatics, He
et al. examine these questions and others in a descriptive case study concerning the software's development, adoption, and use in Côte d'Ivoire. After a full introduction, the authors briefly describe their qualitative methods "to describe the implementation and collaboration around OpenELIS and its supporting activities," followed by discussion of the study results and their implications. The authors conclude that through many different efforts, OpenELIS remains relevant more than a decade later. However, those "planning to adapt and nationally scale [such a] LIS may [need to] consider the importance of HIS workforce development, financial sustainability, and institutionalization of government ownership and technical leadership" through their implementation.
Posted on June 5, 2023
By LabLynx
Journal articles
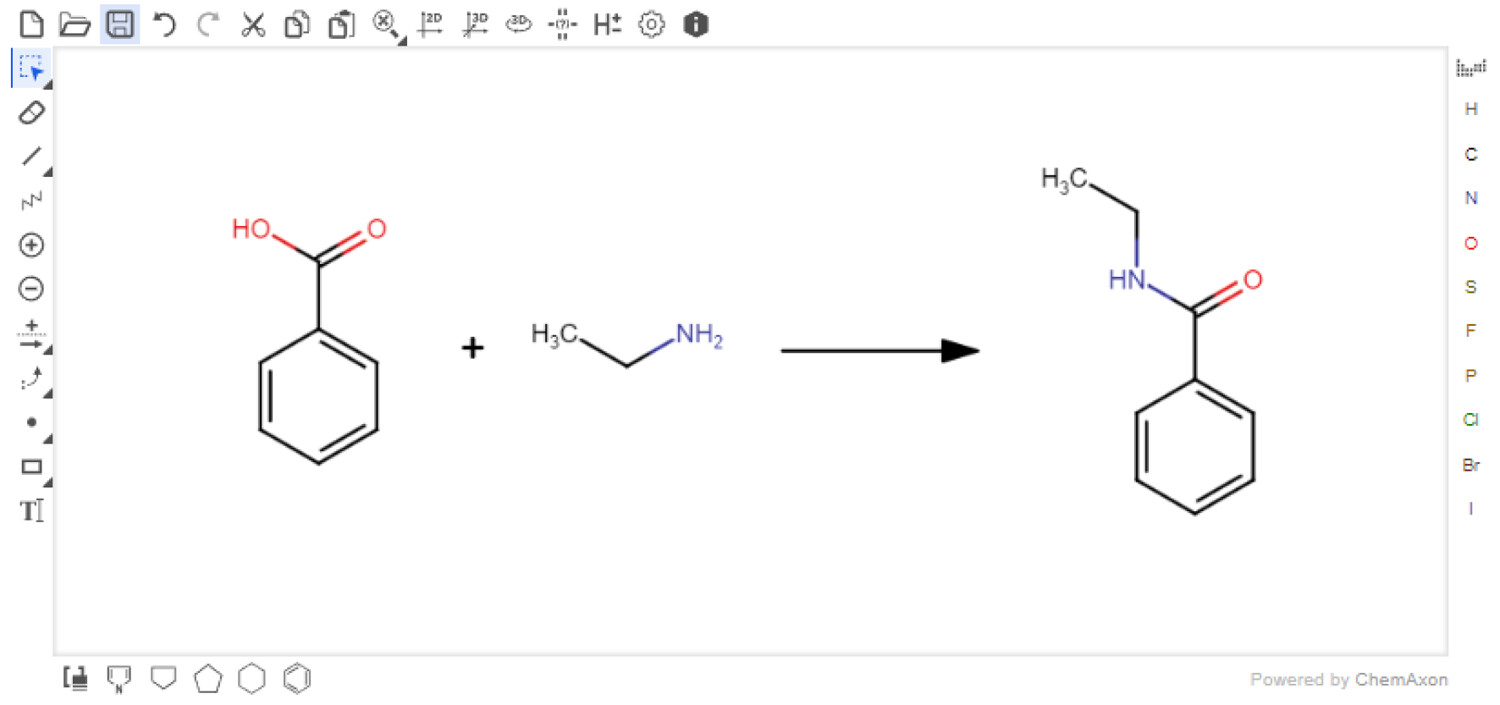
In this 2023 journal article published in the
Journal of Chemical Information and Modeling, Boobier
et al. of University of Nottingham discuss their free, open-source electronic laboratory notebook (ELN) for green and sustainable chemistry practice, AI4Green. The authors state their ELN also "automatically presents the hazards and sustainability of an inputted reaction by calculating sustainability metrics and a color-coded assessment of solvents and reaction conditions." After briefly discussing how it's implemented, the authors discuss the various functions of AI4Green, including its support for workgroups, workbooks, user roles, reaction building, reaction sketching, reaction analysis, exporting, and add-on functionality, such as the Solvent Guide. The authors also discuss how feedback was used during application development. They conclude that AI4Green "combines the practical benefits of an ELN alongside a framework for encouraging green and sustainable chemistry," though more work is to be done to extend its functionality. Regardless, even as-is, "AI4Green provides an exciting initial framework to unite an ELN with sustainable chemistry."
Posted on May 30, 2023
By LabLynx
Journal articles
In this 2023 article published in the Indonesian journal
Matrix: Jurnal Manajemen Teknologi Dan Informatika, Ifriza
et al. present their approach to developing a laboratory-based information system for monitoring the condition and maintenance of instruments at the laboratories of the Faculty of Mathematics and Natural Sciences, State University of Semarang (FMIPA UNNES). After providing a brief introduction, the authors discuss their methodology to developing such a software system, as well as the results, which are explained in terms of the five enterprise architecture planning (EAP) steps used to develop it. The authors conclude that "[b]ased on the results of the study, it can be concluded that the use of laboratory systems for monitoring and maintenance in laboratory management has proven to be very valid."
Posted on May 24, 2023
By LabLynx
Journal articles
In this 2022 paper published in the journal
Complementary Therapies in Medicine, Ennis
et al. discuss the development of a practice-based research network in order to improve the state of understanding of
Cannabis science in the state of Florida, as well as promote future clinical research related to the plant. The authors developed this research network after noting a dearth of such medical marijuana-focused research networks in the state. After providing an introduction, they walk through the steps that led to their Complementary Care Practice-Based Research Network (CC-PBRN), including the methods used in producing usable, de-identified data for research. The authors then summarize the results and address three major challenges in negotiating the "differing foci of academic and business organizations" in developing the network. After noting the strengths and limitations of their network, they conclude that "[t]he data generated by the CC-PBRN can be used to inform and guide patient-centered care, clinical decision-making, and health policy decisions" for medical marijuana patients in Florida.
Posted on May 16, 2023
By LabLynx
Journal articles
In this 2023 paper published in the journal
Accreditation and Quality Assurance, Tziakou
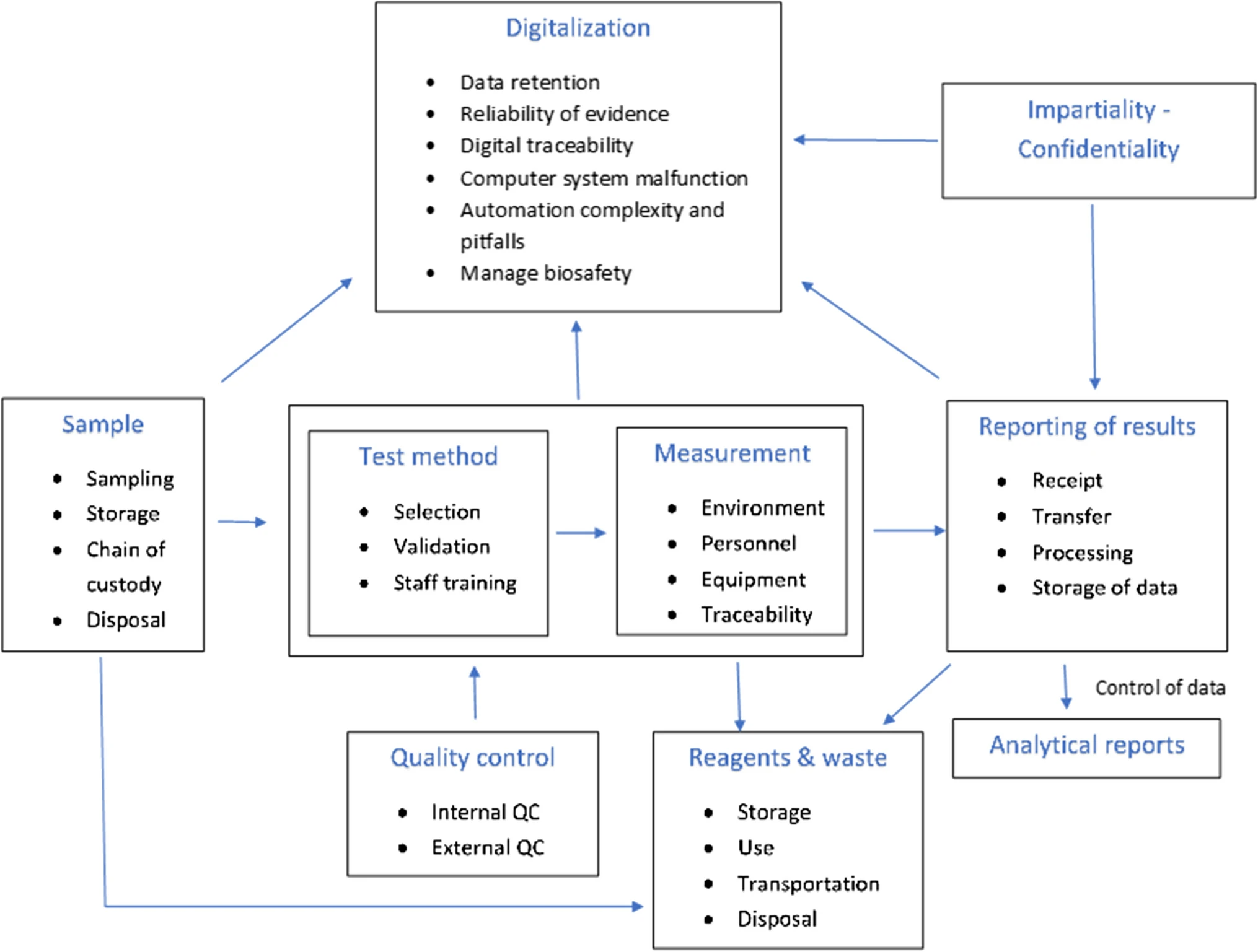
et al. examine the state of risk management as it applies to the modern laboratory environment, tapping into numerous International Organization for Standardization (ISO) standards and a review of the literature on the subject. After a brief introduction, the authors discuss the risk management process and risk assessment techniques for organizations of all types. They then take a deep dive into how those techniques apply to the laboratory environment at every step of the lab's workflow, from sample and reagent management to reporting and digitalization of results. They conclude that given the major sources of risk they identified in the lab, "the laboratory can reduce the risks to a tolerable level when clear procedures, continuous supervision, inspections, timely training and continuous education of its staff, and upgrades of its equipment with systems automation are maintained." They add that "the implementation of risk-based thinking can positively affect the outcome of regular assessments in order to explore opportunities for increasing the effectiveness of the [quality management system] and preventing further negative effects."
Posted on May 9, 2023
By LabLynx
Journal articles
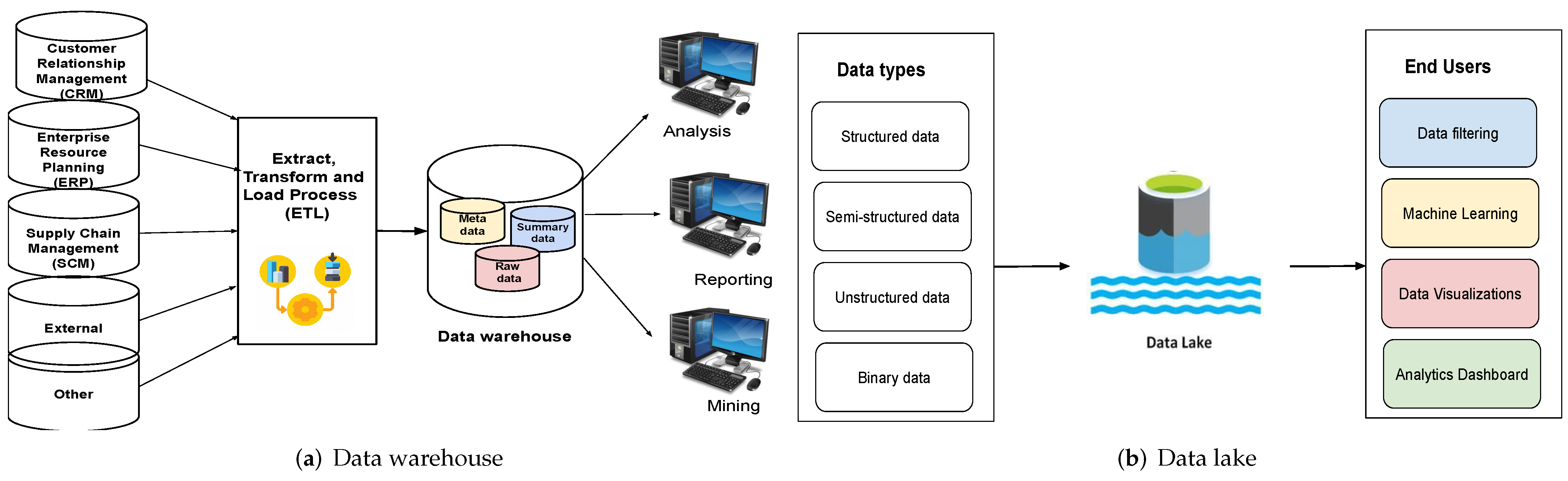
Noting only one known work comparing data warehouses (DWs) with data lakes (DLs), with that article failing to fully address "a comprehensive analysis of both data management schemes by addressing various aspects" in detail, Nambiar and Mundra take to that task in this 2022 article published in
Big Data and Cognitive Computing. After an introduction to the state of DWs and DLs, the authors then discuss the finer points of both data management schemes, while also conducting a literature review on the topic. They then discuss the specifics of architecture regarding DWs and DLs, as well as the data management aspects and useful tools that enhance them. After discussing various challenges and opportunities for the two schemes, the authors conclude that "[d]espite being used interchangeably, they are two distinct storage forms with unique characteristics that serve different purposes."
Posted on May 1, 2023
By LabLynx
Journal articles
While the management of digitally created data and information is largely in the scope of regulators, standards organizations, and company management these days, the non-trivial surplus of analog data and information (e.g., printed tables, field notebooks, photographs, drawings, maps, etc.) and what to do with it still weighs upon on the research community. Older data has long had uses in retrospective and current research, yet few large-scale solutions for making this analog data and information more FAIR (findable, accessible, interoperable, and reusable) have been developed. Kelly
et al. emphasize this in their 2022 research published in the
Data Science Journal and address what they consider should be a set of best practices to managing historic analog data and information. After proving background history on the concerns surrounding older analog data, the authors discuss ways that older analog data is currently and can be used, and they highlight the challenges that come with attempting to reuse such data. They then provide some possible paths forward with a best practice approach, before concluding that "[b]est practices (including selection of metadata schema, developing a data dictionary, describing data collection methods) and policies developed to govern the preservation and dissemination of digital data could serve as an example for developments concerning analog data."
Posted on April 25, 2023
By LabLynx
Journal articles
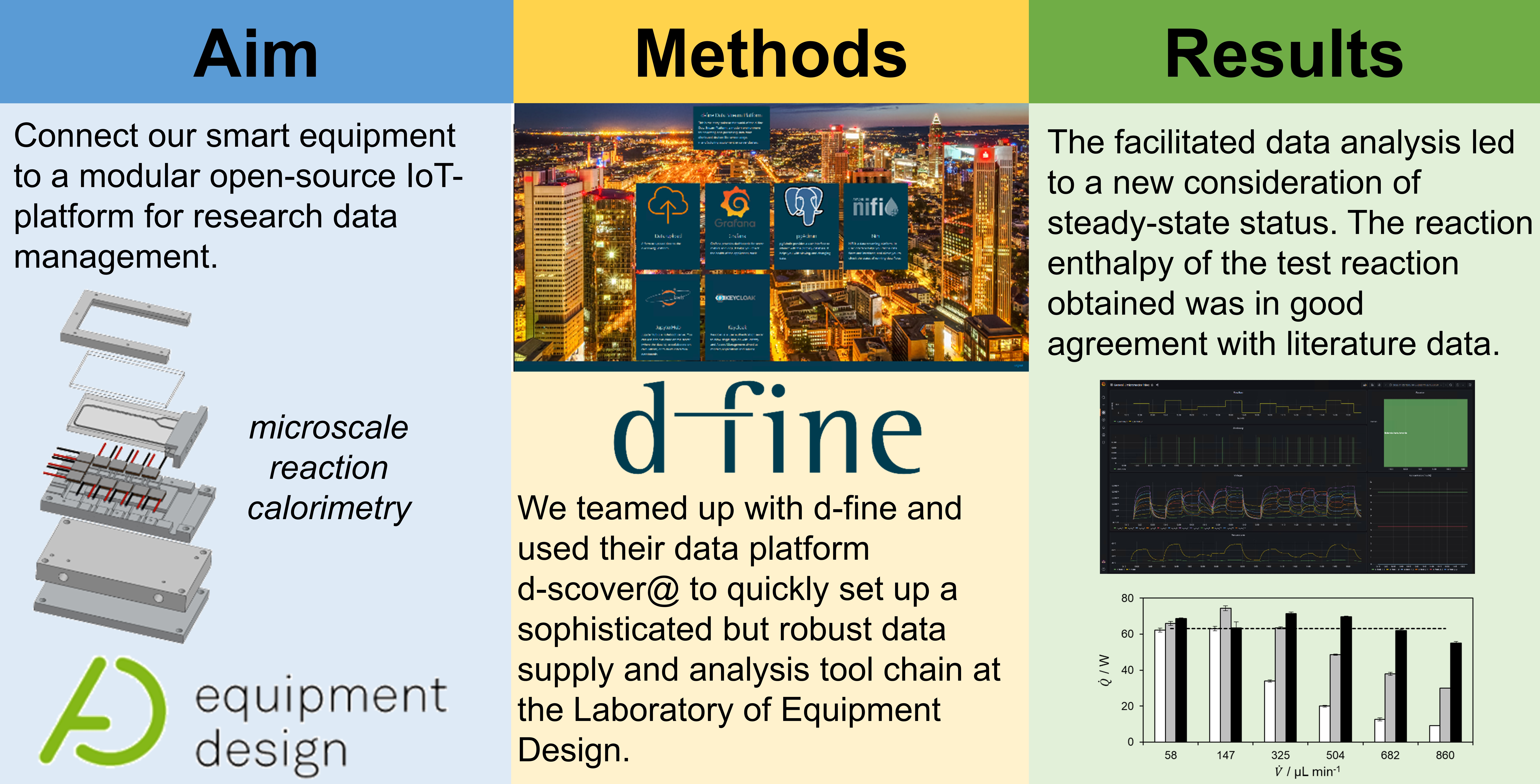
In this 2023 article published in the journal
Processes, Frede
et al. of TU Dortmund University work with analytical consultancy d-fine GmbH to demonstrate experimental workflows of a microscale reaction calorimeter over d-fine's open-source internet of things (IoT) software platform. Their goal: provide an example of how data collection and capture methods can and should be standardized across an automated laboratory. After a brief introduction, the authors present their materials and methods, along with a case study of hydrolysis of acetic anhydride. After presenting the results of their case study, they conclude that "[t]he modular data platform complements the reaction calorimeter very well with additional functionalities," and by adding even more instruments, "[t]his approach will further harmonize data management within our laboratory while better applying FAIR Guiding Principles."
Posted on April 18, 2023
By LabLynx
Journal articles
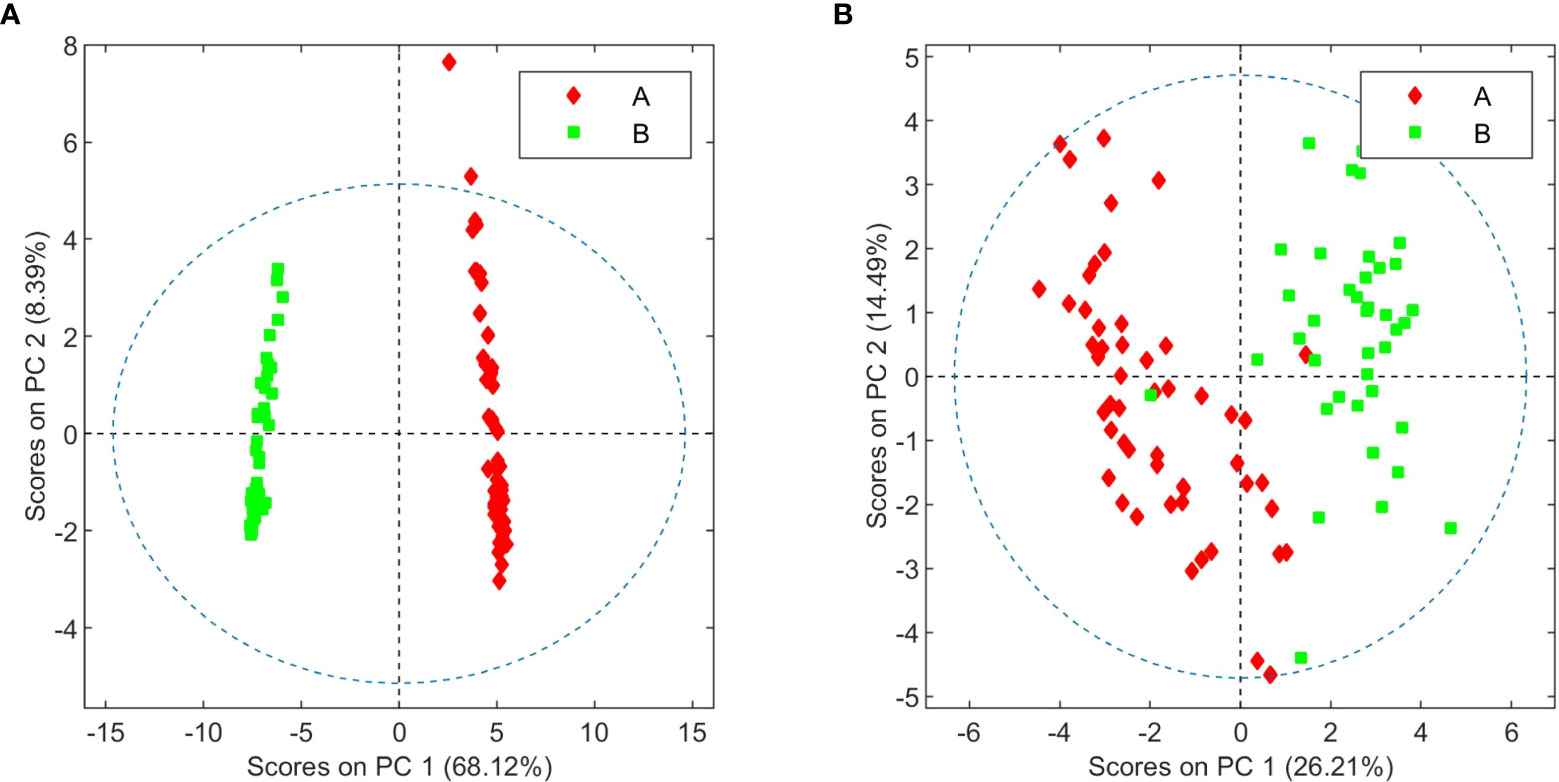
In this 2023 paper published in the journal
Frontiers in Plant Science, Fernández
et al. propose that
Cannabis should be characterized "according to its chemical composition (i.e., its metabolome) and not only its botanical traits," while conducting a series of experiments to back their claim. After an introduction to cannabis and its chemical characterization, the authors describe the materials, methods, and results of their experimentation using metabolomics on high-THCA and high-CBDA
C. sativa chemovars with medicinal potential. They conclude that many "differences between the two varieties beyond their cannabinoid composition" could be found through this experimentation, and that these differences could be "instrumental to breeders in the development of plant varieties agronomically adapted to specific environmental conditions." They also noted that fungal infections changed the metabolome of tested plants, which can better aid in the "classification of healthy and diseased plants."
Posted on April 11, 2023
By LabLynx
Journal articles
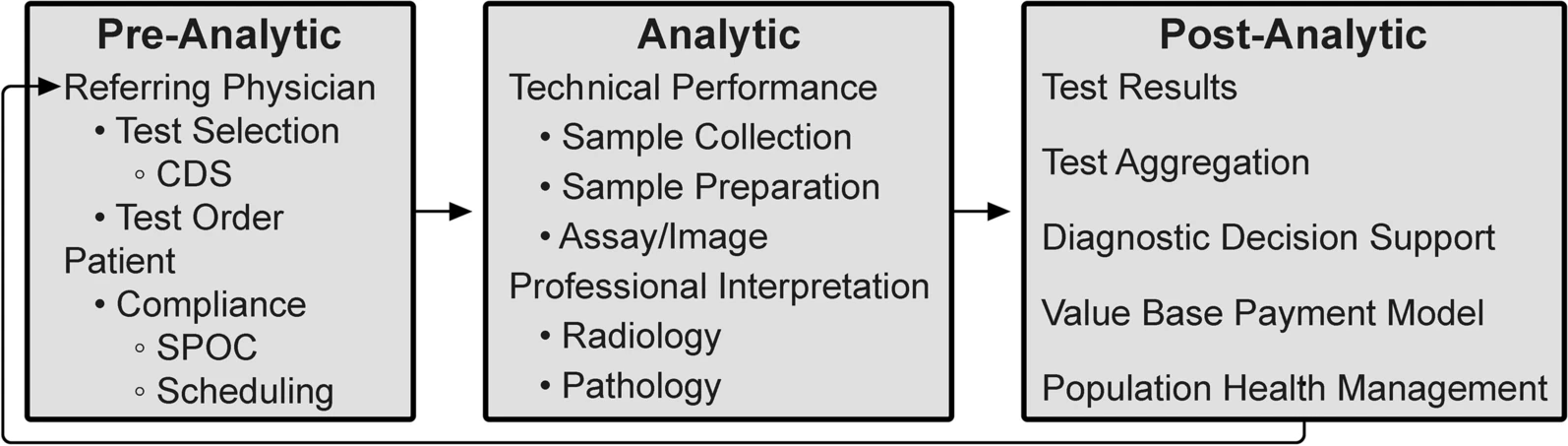
In this 2022 paper published in
Insights into Imaging, Beauchamp
et al. focus in on the promise of "integrative diagnostics" as a means towards aggregating diagnostic data for more meaningful interpretation and contextualization, by extension translating to directly relatable clinical action. Looking specifically at radiology and pathology workflows, the authors describe what integrative diagnostics looks like, how the process should work, and what promise it holds for
in vivo and
in vitro radiology and pathology workflows. The authors turn to 24 different sources in highlighting the clinical potential of integrative diagnostics for a wide variety of diseases, highlighting the added clinical value and improved discovery possible with integrative diagnostics' application. After examining the current state of the art, the authors conclude by making several recommendations towards implementing integrative diagnostics, while also noting that "[c]reating financial models that demonstrate the economic value proposition of [integrative diagnostics] will be a necessary catalyst for change" for the overall health care system.
Posted on April 3, 2023
By LabLynx
Journal articles
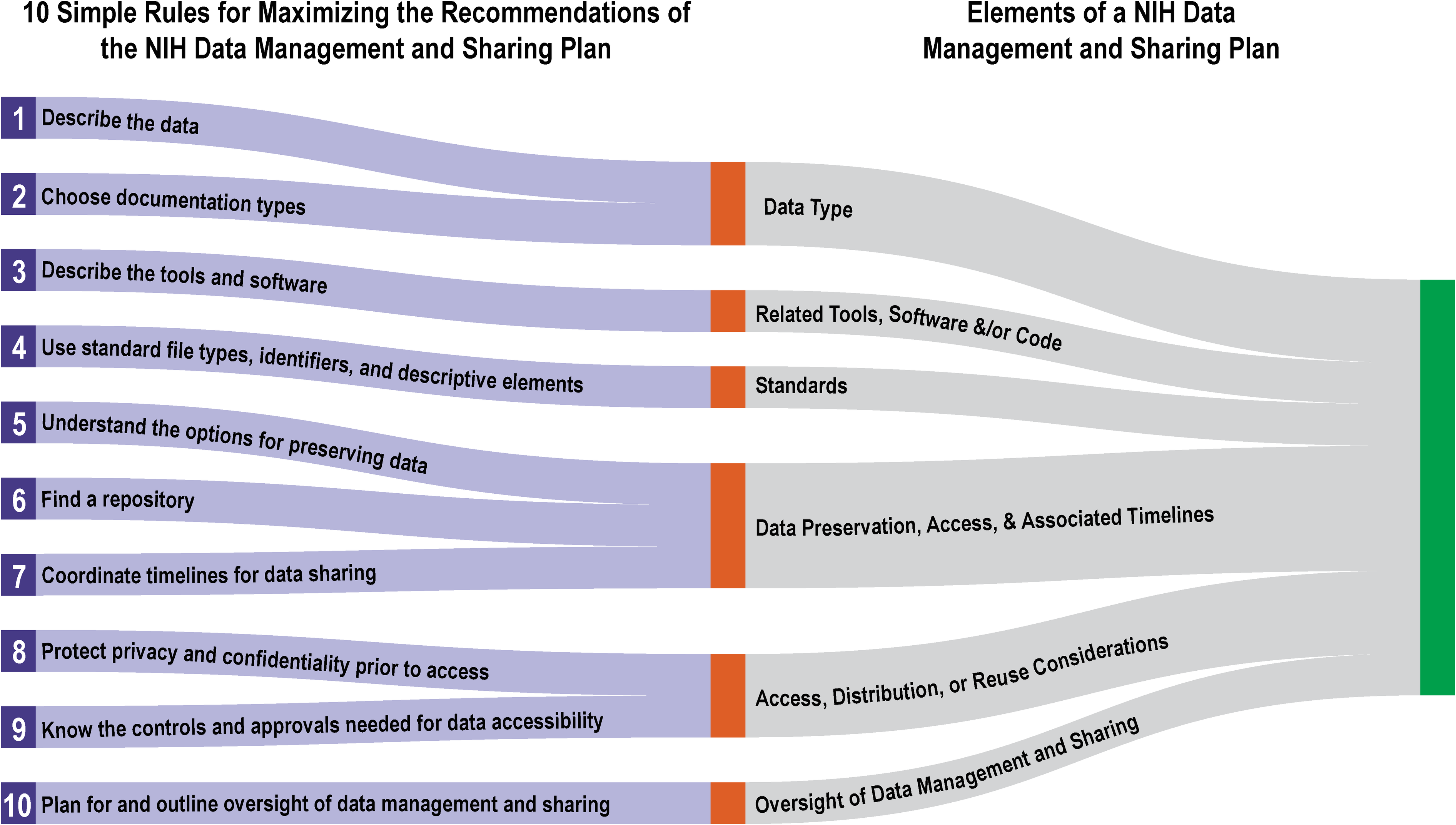
In January 2023, the National Institutes of Health's (NIH's) Policy for Data Management and Sharing came into effect, requiring guidance on elements "for the submission of a data management and sharing plan (DMSP)" for NIH-funded projects. While the NIH created supplementary guidance to developing a DMSP, some ambiguity arguably remained. In this 2022 article published in
PLOS Computational Biology, Gonzales
et al. provide additional insight into the process. After a brief introduction, the authors present 10 helpful tips towards getting the most of a DMSP in complying with the NIH and its requirements. They conclude by examining the various stakeholders involved with the DMSP process and finding that "[g]ood DMSP practices can support better engagement with and accountability to the public who benefit from research."
Posted on March 27, 2023
By LabLynx
Journal articles
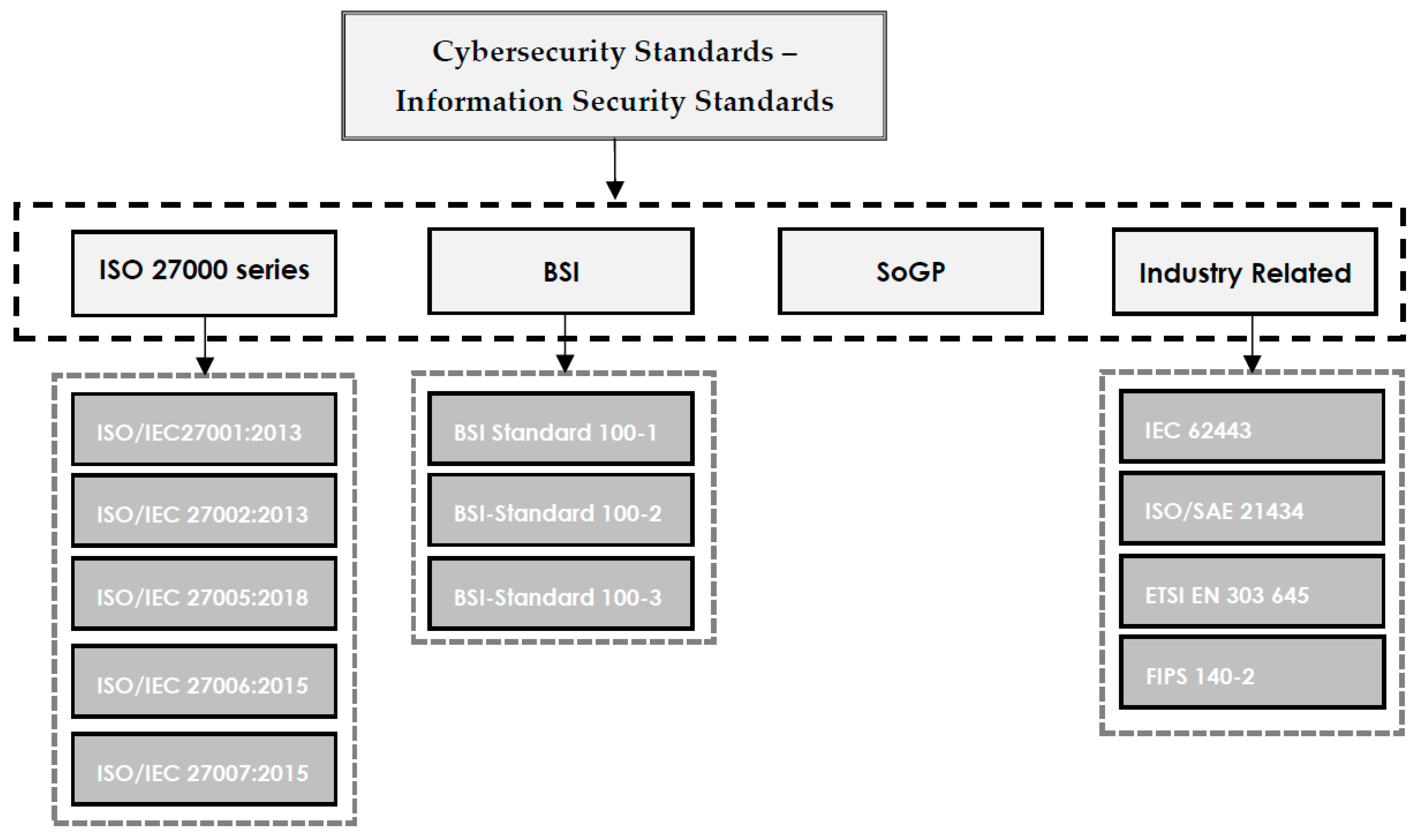
In this 2022 journal article published in
Electronics, University Canada West's Hamed Taherdoost presents a narrative literature review of a variety of cybersecurity standards and frameworks as a means to "help organizations select the cybersecurity standard or framework that best fits their cybersecurity requirements." After providing a brief introduction, Taherdoost speaks generally about cybersecurity standards and frameworks, and what they help organizations of all types achieve with their own cybersecurity. The author then goes into greater detail of those standards and frameworks, as well as their supporting documentation. Taherdoost then presents the methodology for his literature review, followed by a discussion of the results of that review. The author concludes that the presented standards and frameworks help organizations "ensure the security of data against cyber threats," though one standard or framework "may not fulfill all the demands of an organization, and it may be necessary to employ a combination of standards in order to ensure security against cyber threats and data loss."
Posted on March 20, 2023
By LabLynx
Journal articles
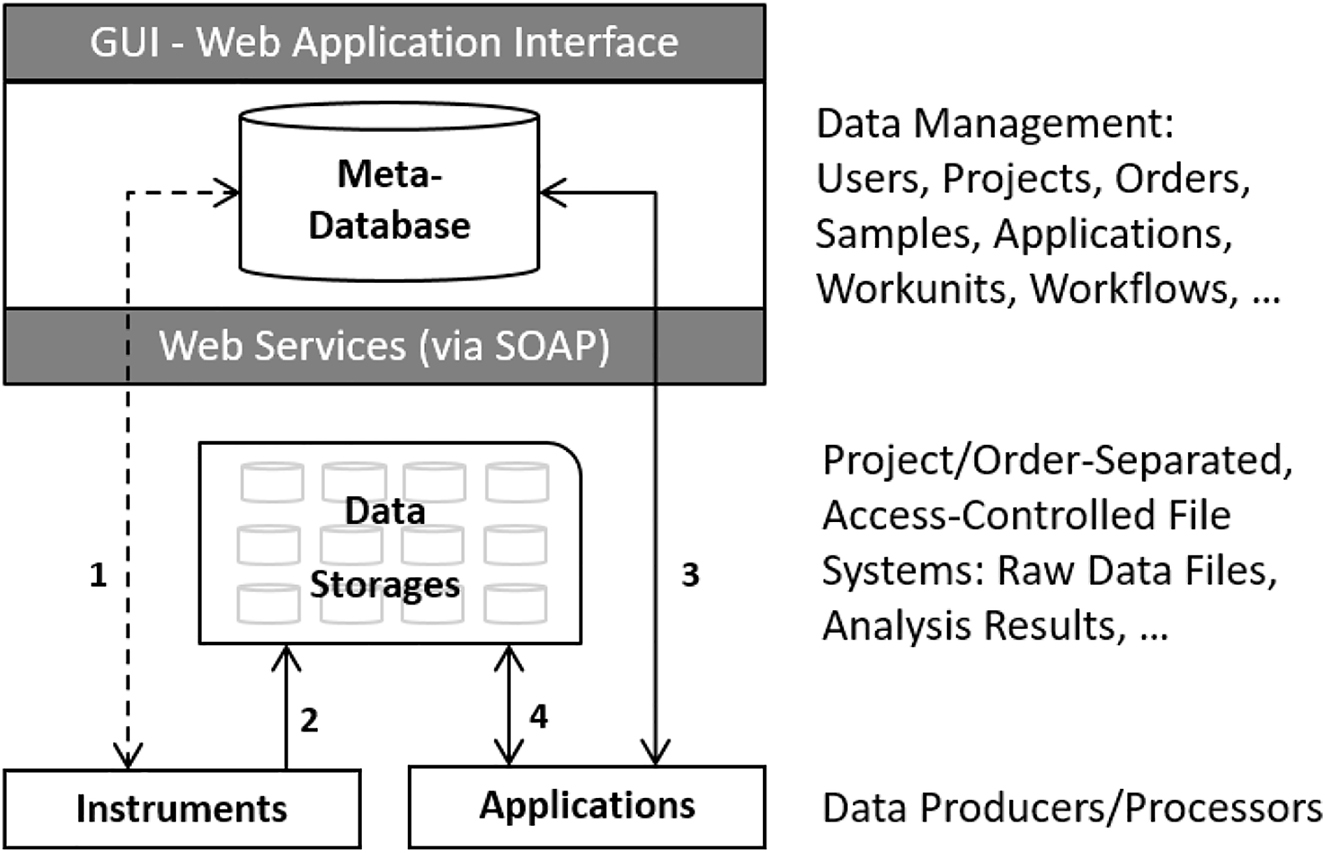
In this 2022 journal article published in
Journal of Integrative Bioinformatics, Panse
et al. discuss the state of research-based core facilities that share research resources across institutions or organizations, in particular in regards to data and information management. The authors note that the "interdisciplinary nature of such scientific projects requires a powerful and flexible platform for data annotation, communication, and exploration," and the authors—associated with the Functional Genomics Center Zurich (FGCZ)—present B-Fabric, their solution to data and information management in such settings. After providing a brief background, the authors discuss the development and application of B-Fabric at FGCZ, along with lessons learned. They conclude that with systems like B-Fabric, "ad-hoc visualizations and analytics can be realized on top of an integrative platform that captures and processes any kind of data and scientific processes." The platform is also able "to decouple metadata management from data processing and visualization components" while also optimizing specific software software environments for different research areas.
Posted on March 13, 2023
By LabLynx
Journal articles
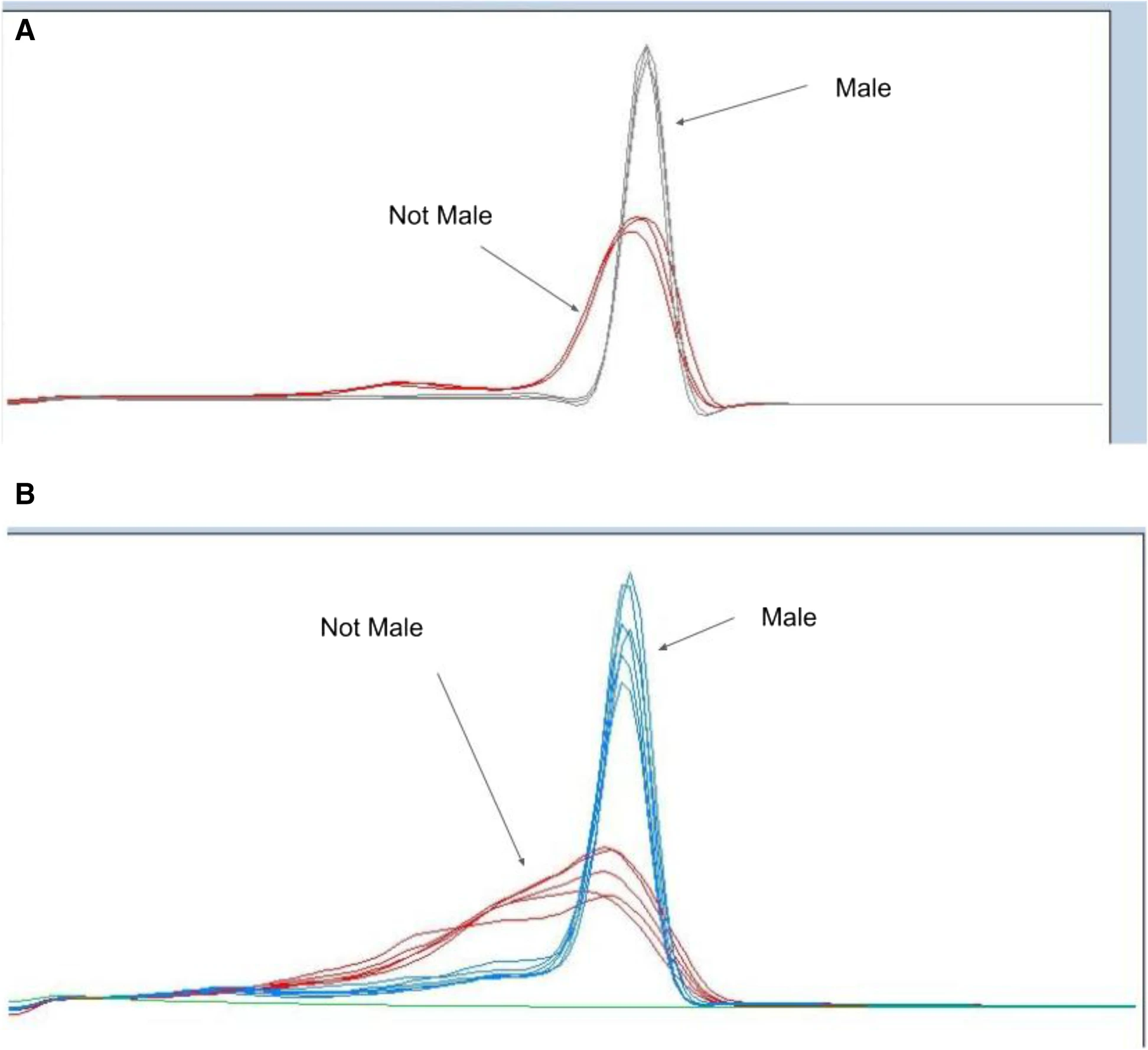
Genotyping of
Cannabis sativa is a useful component of not only gender identification of plants for cultivators but also other types of laboratory testing and research of
Cannabis. Performing such tests in a reliable, cost-effective, and high-performance way has significant value for
Cannabis testing laboratories. In this 2022 journal article, Torres
et al. compare a number of different genotyping techniques, ultimately demonstrating some of that value. After providing background information and their methodology, the authors discuss the results of gender testing using a wide array of genotyping techniques. They conclude that "the multiple methodologies presented here allow for accurate, quick, and cost-effective screening that will enable future development of germplasm and the industry." They add that their "real-time assays can be performed by cannabis testing laboratories performing diagnostic testing, as well as in the field with minimal molecular biology equipment and expertise."
Posted on March 6, 2023
By LabLynx
Journal articles
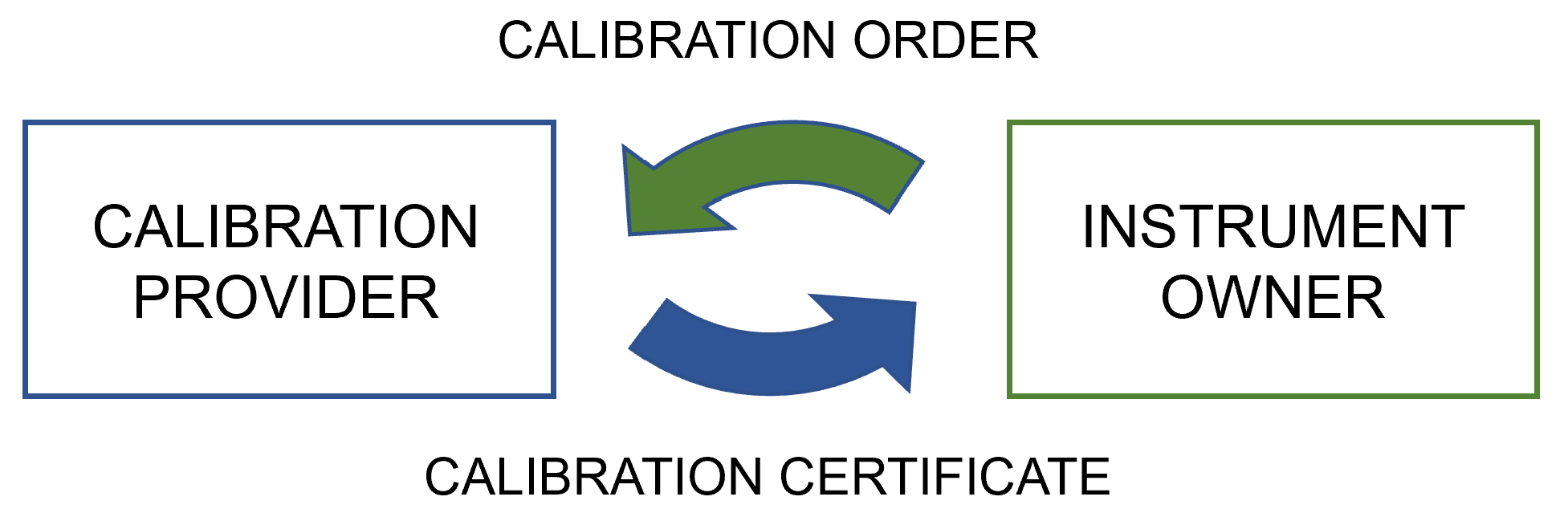
In this 2022 journal article published in
Applied Sciences, Mustapää
et al. turn to the calibration process of the pharmaceutical industry—as well as their regulatory- and standards-based requirements—as inspiration for building a proof-of-concept software platform that better ensures "the preservation of traceability and data integrity" of calibration data. After a lengthy and thorough background to the topic of laboratory calibrations and calibration data management, the authors present their proof of concept software platform, designed "to harmonize the data exchange between the parties of a calibration chain to enable automation of the data management processes in the receiving end." They then discuss the results of their efforts, challenges in advancing such metrology infrastructure, and topics that require further research. They conclude that the real use cases tested on their proof of concept "proved that the multitenant platform is an efficient means of organizing the exchange of [digital calibration certificates]," and that "the system would suit the requirements of other industries as well, providing cost-efficiency through economies of scale."
 In this 2023 article published in the journal Journal of Cannabis Research, Chambers et al. of State University of New York present an analytical method for "the analysis and differentiation of C. sativa plant materials" that, on paper, represent a major boost over more traditional chromatographic methods. After providing some background on those traditional types of analyses, the authors then present their method of using direct analysis in real time high-resolution mass spectrometry (DART-HRMS) with advanced chemometrics, including results associated with use of their method in a controlled laboratory environment. After some discussion, the authors conclude that their developed method "was successfully used to create a prediction model that facilitated rapid high-accuracy differentiation of C. sativa hemp and marijuana plant materials obtained from multiple sources (i.e., commercial, DEA-registered, recreational)," while also noting that "100% accuracy in prediction was observed," making it an important potential future method "for the optimal differentiation of hemp and marijuana."
In this 2023 article published in the journal Journal of Cannabis Research, Chambers et al. of State University of New York present an analytical method for "the analysis and differentiation of C. sativa plant materials" that, on paper, represent a major boost over more traditional chromatographic methods. After providing some background on those traditional types of analyses, the authors then present their method of using direct analysis in real time high-resolution mass spectrometry (DART-HRMS) with advanced chemometrics, including results associated with use of their method in a controlled laboratory environment. After some discussion, the authors conclude that their developed method "was successfully used to create a prediction model that facilitated rapid high-accuracy differentiation of C. sativa hemp and marijuana plant materials obtained from multiple sources (i.e., commercial, DEA-registered, recreational)," while also noting that "100% accuracy in prediction was observed," making it an important potential future method "for the optimal differentiation of hemp and marijuana."
 Just as laboratories of all types managing complex workflows and large amounts of test data can benefit from laboratory information management systems (LIMS), similarly do clinical research groups conducting clinical trials benefit from software-based data and workflow management solutions, including the clinical trial management system (CTMS). From more standardized results and documents to more efficient workflows, such clinical systems are a boon to hospitals and research organizations looking to streamline process management for clinical trials. Highlighting this benefit, Shen et al. present their custom CTMS for the First Affiliated Hospital at Zhejiang University School of Medicine (FAHZU) in this 2023 article published in BMC Medical Informatics and Decision Making. After a thorough explanation of their system development and implementation, as well as discussion concerning its use, the authors conclude that the FAHZU CTMS "fully realizes the whole-process data management of clinical trials from project approval and review management to operational management," while providing "a variety of access methods to complete efficient data integration with [other] clinical business systems."
Just as laboratories of all types managing complex workflows and large amounts of test data can benefit from laboratory information management systems (LIMS), similarly do clinical research groups conducting clinical trials benefit from software-based data and workflow management solutions, including the clinical trial management system (CTMS). From more standardized results and documents to more efficient workflows, such clinical systems are a boon to hospitals and research organizations looking to streamline process management for clinical trials. Highlighting this benefit, Shen et al. present their custom CTMS for the First Affiliated Hospital at Zhejiang University School of Medicine (FAHZU) in this 2023 article published in BMC Medical Informatics and Decision Making. After a thorough explanation of their system development and implementation, as well as discussion concerning its use, the authors conclude that the FAHZU CTMS "fully realizes the whole-process data management of clinical trials from project approval and review management to operational management," while providing "a variety of access methods to complete efficient data integration with [other] clinical business systems."
 In this 2023 article published in the Journal of Pathology Informatics, Pablo et al. of the University of Washington School of Medicine present their work on developing a web-based application for better supporting the complex workflows of labs conducting pain-management-related toxicology and pathology testing. Noting a lack of support of these workflows among traditional laboratory information systems (LISs), the authors describe their approach to developing a system to meet their needs. After providing background and discussing the development approach, the authors present the results of their system implementation and performance analysis. After some discussion, the authors conclude that the "implementation of a purpose-built application to support reflex and interpretation workflows in a clinical pathology practice has led to a significant improvement in laboratory efficiency," adding that their custom, purpose-built application is able to "reduce staff burnout, reduce transcription errors, and allow staff to focus on more critical issues around quality."
In this 2023 article published in the Journal of Pathology Informatics, Pablo et al. of the University of Washington School of Medicine present their work on developing a web-based application for better supporting the complex workflows of labs conducting pain-management-related toxicology and pathology testing. Noting a lack of support of these workflows among traditional laboratory information systems (LISs), the authors describe their approach to developing a system to meet their needs. After providing background and discussing the development approach, the authors present the results of their system implementation and performance analysis. After some discussion, the authors conclude that the "implementation of a purpose-built application to support reflex and interpretation workflows in a clinical pathology practice has led to a significant improvement in laboratory efficiency," adding that their custom, purpose-built application is able to "reduce staff burnout, reduce transcription errors, and allow staff to focus on more critical issues around quality."
 In this 2023 article published in the journal Smart Agricultural Technology, Sutton et al. propose an automated method for analyzing the trichomes of the Cannabis inflorescences for their cultivation maturity. Noting "no scientifically based methods to predict maturation of inflorescences" in the literature, the authors sought out a computational method able to " extract trichome phenotype and morphology metrics during Cannabis flower development from macroscopic photographs." After providing a brief introduction and a discussion of related work, the researchers discuss their materials, analytical methods, and data collection approach, followed by a lengthy explanation of their experimental results. The authors conclude that through their methods, "the observed relationship between trichome gland head diameter and morphological metrics indicates the feasibility of automatic quality assurance software that can determine how flower maturation is proceeding and if strain-specific potential may be attained." They add that further expansion on their work may produce real practical solutions for cultivators gauging the harvest-readiness of their Cannabis plants.
In this 2023 article published in the journal Smart Agricultural Technology, Sutton et al. propose an automated method for analyzing the trichomes of the Cannabis inflorescences for their cultivation maturity. Noting "no scientifically based methods to predict maturation of inflorescences" in the literature, the authors sought out a computational method able to " extract trichome phenotype and morphology metrics during Cannabis flower development from macroscopic photographs." After providing a brief introduction and a discussion of related work, the researchers discuss their materials, analytical methods, and data collection approach, followed by a lengthy explanation of their experimental results. The authors conclude that through their methods, "the observed relationship between trichome gland head diameter and morphological metrics indicates the feasibility of automatic quality assurance software that can determine how flower maturation is proceeding and if strain-specific potential may be attained." They add that further expansion on their work may produce real practical solutions for cultivators gauging the harvest-readiness of their Cannabis plants.
 In this 2022 journal article published in Frontiers in Bioinformatics, Davis and Jorgensen of the Howard Hughes Medical Institute and School of Biological Sciences at University of Utah discuss their free, multi-platform software for visualizing, designing, and presenting DNA materials and sequences. Noting that many such applications are out of reach of the small academic or teaching laboratory, the authors have developed and maintained A Plasmid Editor (ApE) for over 17 years, becoming a robust and versatile tool for molecular biologists. After introducing the software in the context of other such software, both free and commercial, they discuss their approach to developing the software over the years, followed by an extensive review of its features and functions. The authors conclude that ApE continues to be a solid " platform for creating visually appealing linear and circular plasmid maps," as well as simulate molecular techniques, adding "that many of its features and user interfaces have been inspired by input from users requesting new or modified functionality" over the years.
In this 2022 journal article published in Frontiers in Bioinformatics, Davis and Jorgensen of the Howard Hughes Medical Institute and School of Biological Sciences at University of Utah discuss their free, multi-platform software for visualizing, designing, and presenting DNA materials and sequences. Noting that many such applications are out of reach of the small academic or teaching laboratory, the authors have developed and maintained A Plasmid Editor (ApE) for over 17 years, becoming a robust and versatile tool for molecular biologists. After introducing the software in the context of other such software, both free and commercial, they discuss their approach to developing the software over the years, followed by an extensive review of its features and functions. The authors conclude that ApE continues to be a solid " platform for creating visually appealing linear and circular plasmid maps," as well as simulate molecular techniques, adding "that many of its features and user interfaces have been inspired by input from users requesting new or modified functionality" over the years.
 OpenELIS, a free open-source laboratory information system (LIS) for public health laboratories, has been around since 2012 and continue to garner new adopters. But what of the software more than a decade later? Is it still relevant and updated, and has the software demonstrated elements of sustainability for those who have installed it? In this 2023 paper published in the International Journal of Medical Informatics, He et al. examine these questions and others in a descriptive case study concerning the software's development, adoption, and use in Côte d'Ivoire. After a full introduction, the authors briefly describe their qualitative methods "to describe the implementation and collaboration around OpenELIS and its supporting activities," followed by discussion of the study results and their implications. The authors conclude that through many different efforts, OpenELIS remains relevant more than a decade later. However, those "planning to adapt and nationally scale [such a] LIS may [need to] consider the importance of HIS workforce development, financial sustainability, and institutionalization of government ownership and technical leadership" through their implementation.
OpenELIS, a free open-source laboratory information system (LIS) for public health laboratories, has been around since 2012 and continue to garner new adopters. But what of the software more than a decade later? Is it still relevant and updated, and has the software demonstrated elements of sustainability for those who have installed it? In this 2023 paper published in the International Journal of Medical Informatics, He et al. examine these questions and others in a descriptive case study concerning the software's development, adoption, and use in Côte d'Ivoire. After a full introduction, the authors briefly describe their qualitative methods "to describe the implementation and collaboration around OpenELIS and its supporting activities," followed by discussion of the study results and their implications. The authors conclude that through many different efforts, OpenELIS remains relevant more than a decade later. However, those "planning to adapt and nationally scale [such a] LIS may [need to] consider the importance of HIS workforce development, financial sustainability, and institutionalization of government ownership and technical leadership" through their implementation.
 In this 2023 journal article published in the Journal of Chemical Information and Modeling, Boobier et al. of University of Nottingham discuss their free, open-source electronic laboratory notebook (ELN) for green and sustainable chemistry practice, AI4Green. The authors state their ELN also "automatically presents the hazards and sustainability of an inputted reaction by calculating sustainability metrics and a color-coded assessment of solvents and reaction conditions." After briefly discussing how it's implemented, the authors discuss the various functions of AI4Green, including its support for workgroups, workbooks, user roles, reaction building, reaction sketching, reaction analysis, exporting, and add-on functionality, such as the Solvent Guide. The authors also discuss how feedback was used during application development. They conclude that AI4Green "combines the practical benefits of an ELN alongside a framework for encouraging green and sustainable chemistry," though more work is to be done to extend its functionality. Regardless, even as-is, "AI4Green provides an exciting initial framework to unite an ELN with sustainable chemistry."
In this 2023 journal article published in the Journal of Chemical Information and Modeling, Boobier et al. of University of Nottingham discuss their free, open-source electronic laboratory notebook (ELN) for green and sustainable chemistry practice, AI4Green. The authors state their ELN also "automatically presents the hazards and sustainability of an inputted reaction by calculating sustainability metrics and a color-coded assessment of solvents and reaction conditions." After briefly discussing how it's implemented, the authors discuss the various functions of AI4Green, including its support for workgroups, workbooks, user roles, reaction building, reaction sketching, reaction analysis, exporting, and add-on functionality, such as the Solvent Guide. The authors also discuss how feedback was used during application development. They conclude that AI4Green "combines the practical benefits of an ELN alongside a framework for encouraging green and sustainable chemistry," though more work is to be done to extend its functionality. Regardless, even as-is, "AI4Green provides an exciting initial framework to unite an ELN with sustainable chemistry."
 In this 2023 article published in the Indonesian journal Matrix: Jurnal Manajemen Teknologi Dan Informatika, Ifriza et al. present their approach to developing a laboratory-based information system for monitoring the condition and maintenance of instruments at the laboratories of the Faculty of Mathematics and Natural Sciences, State University of Semarang (FMIPA UNNES). After providing a brief introduction, the authors discuss their methodology to developing such a software system, as well as the results, which are explained in terms of the five enterprise architecture planning (EAP) steps used to develop it. The authors conclude that "[b]ased on the results of the study, it can be concluded that the use of laboratory systems for monitoring and maintenance in laboratory management has proven to be very valid."
In this 2023 article published in the Indonesian journal Matrix: Jurnal Manajemen Teknologi Dan Informatika, Ifriza et al. present their approach to developing a laboratory-based information system for monitoring the condition and maintenance of instruments at the laboratories of the Faculty of Mathematics and Natural Sciences, State University of Semarang (FMIPA UNNES). After providing a brief introduction, the authors discuss their methodology to developing such a software system, as well as the results, which are explained in terms of the five enterprise architecture planning (EAP) steps used to develop it. The authors conclude that "[b]ased on the results of the study, it can be concluded that the use of laboratory systems for monitoring and maintenance in laboratory management has proven to be very valid."
 In this 2022 paper published in the journal Complementary Therapies in Medicine, Ennis et al. discuss the development of a practice-based research network in order to improve the state of understanding of Cannabis science in the state of Florida, as well as promote future clinical research related to the plant. The authors developed this research network after noting a dearth of such medical marijuana-focused research networks in the state. After providing an introduction, they walk through the steps that led to their Complementary Care Practice-Based Research Network (CC-PBRN), including the methods used in producing usable, de-identified data for research. The authors then summarize the results and address three major challenges in negotiating the "differing foci of academic and business organizations" in developing the network. After noting the strengths and limitations of their network, they conclude that "[t]he data generated by the CC-PBRN can be used to inform and guide patient-centered care, clinical decision-making, and health policy decisions" for medical marijuana patients in Florida.
In this 2022 paper published in the journal Complementary Therapies in Medicine, Ennis et al. discuss the development of a practice-based research network in order to improve the state of understanding of Cannabis science in the state of Florida, as well as promote future clinical research related to the plant. The authors developed this research network after noting a dearth of such medical marijuana-focused research networks in the state. After providing an introduction, they walk through the steps that led to their Complementary Care Practice-Based Research Network (CC-PBRN), including the methods used in producing usable, de-identified data for research. The authors then summarize the results and address three major challenges in negotiating the "differing foci of academic and business organizations" in developing the network. After noting the strengths and limitations of their network, they conclude that "[t]he data generated by the CC-PBRN can be used to inform and guide patient-centered care, clinical decision-making, and health policy decisions" for medical marijuana patients in Florida.
 In this 2023 paper published in the journal Accreditation and Quality Assurance, Tziakou et al. examine the state of risk management as it applies to the modern laboratory environment, tapping into numerous International Organization for Standardization (ISO) standards and a review of the literature on the subject. After a brief introduction, the authors discuss the risk management process and risk assessment techniques for organizations of all types. They then take a deep dive into how those techniques apply to the laboratory environment at every step of the lab's workflow, from sample and reagent management to reporting and digitalization of results. They conclude that given the major sources of risk they identified in the lab, "the laboratory can reduce the risks to a tolerable level when clear procedures, continuous supervision, inspections, timely training and continuous education of its staff, and upgrades of its equipment with systems automation are maintained." They add that "the implementation of risk-based thinking can positively affect the outcome of regular assessments in order to explore opportunities for increasing the effectiveness of the [quality management system] and preventing further negative effects."
In this 2023 paper published in the journal Accreditation and Quality Assurance, Tziakou et al. examine the state of risk management as it applies to the modern laboratory environment, tapping into numerous International Organization for Standardization (ISO) standards and a review of the literature on the subject. After a brief introduction, the authors discuss the risk management process and risk assessment techniques for organizations of all types. They then take a deep dive into how those techniques apply to the laboratory environment at every step of the lab's workflow, from sample and reagent management to reporting and digitalization of results. They conclude that given the major sources of risk they identified in the lab, "the laboratory can reduce the risks to a tolerable level when clear procedures, continuous supervision, inspections, timely training and continuous education of its staff, and upgrades of its equipment with systems automation are maintained." They add that "the implementation of risk-based thinking can positively affect the outcome of regular assessments in order to explore opportunities for increasing the effectiveness of the [quality management system] and preventing further negative effects."
 Noting only one known work comparing data warehouses (DWs) with data lakes (DLs), with that article failing to fully address "a comprehensive analysis of both data management schemes by addressing various aspects" in detail, Nambiar and Mundra take to that task in this 2022 article published in Big Data and Cognitive Computing. After an introduction to the state of DWs and DLs, the authors then discuss the finer points of both data management schemes, while also conducting a literature review on the topic. They then discuss the specifics of architecture regarding DWs and DLs, as well as the data management aspects and useful tools that enhance them. After discussing various challenges and opportunities for the two schemes, the authors conclude that "[d]espite being used interchangeably, they are two distinct storage forms with unique characteristics that serve different purposes."
Noting only one known work comparing data warehouses (DWs) with data lakes (DLs), with that article failing to fully address "a comprehensive analysis of both data management schemes by addressing various aspects" in detail, Nambiar and Mundra take to that task in this 2022 article published in Big Data and Cognitive Computing. After an introduction to the state of DWs and DLs, the authors then discuss the finer points of both data management schemes, while also conducting a literature review on the topic. They then discuss the specifics of architecture regarding DWs and DLs, as well as the data management aspects and useful tools that enhance them. After discussing various challenges and opportunities for the two schemes, the authors conclude that "[d]espite being used interchangeably, they are two distinct storage forms with unique characteristics that serve different purposes."
 While the management of digitally created data and information is largely in the scope of regulators, standards organizations, and company management these days, the non-trivial surplus of analog data and information (e.g., printed tables, field notebooks, photographs, drawings, maps, etc.) and what to do with it still weighs upon on the research community. Older data has long had uses in retrospective and current research, yet few large-scale solutions for making this analog data and information more FAIR (findable, accessible, interoperable, and reusable) have been developed. Kelly et al. emphasize this in their 2022 research published in the Data Science Journal and address what they consider should be a set of best practices to managing historic analog data and information. After proving background history on the concerns surrounding older analog data, the authors discuss ways that older analog data is currently and can be used, and they highlight the challenges that come with attempting to reuse such data. They then provide some possible paths forward with a best practice approach, before concluding that "[b]est practices (including selection of metadata schema, developing a data dictionary, describing data collection methods) and policies developed to govern the preservation and dissemination of digital data could serve as an example for developments concerning analog data."
While the management of digitally created data and information is largely in the scope of regulators, standards organizations, and company management these days, the non-trivial surplus of analog data and information (e.g., printed tables, field notebooks, photographs, drawings, maps, etc.) and what to do with it still weighs upon on the research community. Older data has long had uses in retrospective and current research, yet few large-scale solutions for making this analog data and information more FAIR (findable, accessible, interoperable, and reusable) have been developed. Kelly et al. emphasize this in their 2022 research published in the Data Science Journal and address what they consider should be a set of best practices to managing historic analog data and information. After proving background history on the concerns surrounding older analog data, the authors discuss ways that older analog data is currently and can be used, and they highlight the challenges that come with attempting to reuse such data. They then provide some possible paths forward with a best practice approach, before concluding that "[b]est practices (including selection of metadata schema, developing a data dictionary, describing data collection methods) and policies developed to govern the preservation and dissemination of digital data could serve as an example for developments concerning analog data."
 In this 2023 article published in the journal Processes, Frede et al. of TU Dortmund University work with analytical consultancy d-fine GmbH to demonstrate experimental workflows of a microscale reaction calorimeter over d-fine's open-source internet of things (IoT) software platform. Their goal: provide an example of how data collection and capture methods can and should be standardized across an automated laboratory. After a brief introduction, the authors present their materials and methods, along with a case study of hydrolysis of acetic anhydride. After presenting the results of their case study, they conclude that "[t]he modular data platform complements the reaction calorimeter very well with additional functionalities," and by adding even more instruments, "[t]his approach will further harmonize data management within our laboratory while better applying FAIR Guiding Principles."
In this 2023 article published in the journal Processes, Frede et al. of TU Dortmund University work with analytical consultancy d-fine GmbH to demonstrate experimental workflows of a microscale reaction calorimeter over d-fine's open-source internet of things (IoT) software platform. Their goal: provide an example of how data collection and capture methods can and should be standardized across an automated laboratory. After a brief introduction, the authors present their materials and methods, along with a case study of hydrolysis of acetic anhydride. After presenting the results of their case study, they conclude that "[t]he modular data platform complements the reaction calorimeter very well with additional functionalities," and by adding even more instruments, "[t]his approach will further harmonize data management within our laboratory while better applying FAIR Guiding Principles."
 In this 2023 paper published in the journal Frontiers in Plant Science, Fernández et al. propose that Cannabis should be characterized "according to its chemical composition (i.e., its metabolome) and not only its botanical traits," while conducting a series of experiments to back their claim. After an introduction to cannabis and its chemical characterization, the authors describe the materials, methods, and results of their experimentation using metabolomics on high-THCA and high-CBDA C. sativa chemovars with medicinal potential. They conclude that many "differences between the two varieties beyond their cannabinoid composition" could be found through this experimentation, and that these differences could be "instrumental to breeders in the development of plant varieties agronomically adapted to specific environmental conditions." They also noted that fungal infections changed the metabolome of tested plants, which can better aid in the "classification of healthy and diseased plants."
In this 2023 paper published in the journal Frontiers in Plant Science, Fernández et al. propose that Cannabis should be characterized "according to its chemical composition (i.e., its metabolome) and not only its botanical traits," while conducting a series of experiments to back their claim. After an introduction to cannabis and its chemical characterization, the authors describe the materials, methods, and results of their experimentation using metabolomics on high-THCA and high-CBDA C. sativa chemovars with medicinal potential. They conclude that many "differences between the two varieties beyond their cannabinoid composition" could be found through this experimentation, and that these differences could be "instrumental to breeders in the development of plant varieties agronomically adapted to specific environmental conditions." They also noted that fungal infections changed the metabolome of tested plants, which can better aid in the "classification of healthy and diseased plants."
 In this 2022 paper published in Insights into Imaging, Beauchamp et al. focus in on the promise of "integrative diagnostics" as a means towards aggregating diagnostic data for more meaningful interpretation and contextualization, by extension translating to directly relatable clinical action. Looking specifically at radiology and pathology workflows, the authors describe what integrative diagnostics looks like, how the process should work, and what promise it holds for in vivo and in vitro radiology and pathology workflows. The authors turn to 24 different sources in highlighting the clinical potential of integrative diagnostics for a wide variety of diseases, highlighting the added clinical value and improved discovery possible with integrative diagnostics' application. After examining the current state of the art, the authors conclude by making several recommendations towards implementing integrative diagnostics, while also noting that "[c]reating financial models that demonstrate the economic value proposition of [integrative diagnostics] will be a necessary catalyst for change" for the overall health care system.
In this 2022 paper published in Insights into Imaging, Beauchamp et al. focus in on the promise of "integrative diagnostics" as a means towards aggregating diagnostic data for more meaningful interpretation and contextualization, by extension translating to directly relatable clinical action. Looking specifically at radiology and pathology workflows, the authors describe what integrative diagnostics looks like, how the process should work, and what promise it holds for in vivo and in vitro radiology and pathology workflows. The authors turn to 24 different sources in highlighting the clinical potential of integrative diagnostics for a wide variety of diseases, highlighting the added clinical value and improved discovery possible with integrative diagnostics' application. After examining the current state of the art, the authors conclude by making several recommendations towards implementing integrative diagnostics, while also noting that "[c]reating financial models that demonstrate the economic value proposition of [integrative diagnostics] will be a necessary catalyst for change" for the overall health care system.
 In January 2023, the National Institutes of Health's (NIH's) Policy for Data Management and Sharing came into effect, requiring guidance on elements "for the submission of a data management and sharing plan (DMSP)" for NIH-funded projects. While the NIH created supplementary guidance to developing a DMSP, some ambiguity arguably remained. In this 2022 article published in PLOS Computational Biology, Gonzales et al. provide additional insight into the process. After a brief introduction, the authors present 10 helpful tips towards getting the most of a DMSP in complying with the NIH and its requirements. They conclude by examining the various stakeholders involved with the DMSP process and finding that "[g]ood DMSP practices can support better engagement with and accountability to the public who benefit from research."
In January 2023, the National Institutes of Health's (NIH's) Policy for Data Management and Sharing came into effect, requiring guidance on elements "for the submission of a data management and sharing plan (DMSP)" for NIH-funded projects. While the NIH created supplementary guidance to developing a DMSP, some ambiguity arguably remained. In this 2022 article published in PLOS Computational Biology, Gonzales et al. provide additional insight into the process. After a brief introduction, the authors present 10 helpful tips towards getting the most of a DMSP in complying with the NIH and its requirements. They conclude by examining the various stakeholders involved with the DMSP process and finding that "[g]ood DMSP practices can support better engagement with and accountability to the public who benefit from research."
 In this 2022 journal article published in Electronics, University Canada West's Hamed Taherdoost presents a narrative literature review of a variety of cybersecurity standards and frameworks as a means to "help organizations select the cybersecurity standard or framework that best fits their cybersecurity requirements." After providing a brief introduction, Taherdoost speaks generally about cybersecurity standards and frameworks, and what they help organizations of all types achieve with their own cybersecurity. The author then goes into greater detail of those standards and frameworks, as well as their supporting documentation. Taherdoost then presents the methodology for his literature review, followed by a discussion of the results of that review. The author concludes that the presented standards and frameworks help organizations "ensure the security of data against cyber threats," though one standard or framework "may not fulfill all the demands of an organization, and it may be necessary to employ a combination of standards in order to ensure security against cyber threats and data loss."
In this 2022 journal article published in Electronics, University Canada West's Hamed Taherdoost presents a narrative literature review of a variety of cybersecurity standards and frameworks as a means to "help organizations select the cybersecurity standard or framework that best fits their cybersecurity requirements." After providing a brief introduction, Taherdoost speaks generally about cybersecurity standards and frameworks, and what they help organizations of all types achieve with their own cybersecurity. The author then goes into greater detail of those standards and frameworks, as well as their supporting documentation. Taherdoost then presents the methodology for his literature review, followed by a discussion of the results of that review. The author concludes that the presented standards and frameworks help organizations "ensure the security of data against cyber threats," though one standard or framework "may not fulfill all the demands of an organization, and it may be necessary to employ a combination of standards in order to ensure security against cyber threats and data loss."
 In this 2022 journal article published in Journal of Integrative Bioinformatics, Panse et al. discuss the state of research-based core facilities that share research resources across institutions or organizations, in particular in regards to data and information management. The authors note that the "interdisciplinary nature of such scientific projects requires a powerful and flexible platform for data annotation, communication, and exploration," and the authors—associated with the Functional Genomics Center Zurich (FGCZ)—present B-Fabric, their solution to data and information management in such settings. After providing a brief background, the authors discuss the development and application of B-Fabric at FGCZ, along with lessons learned. They conclude that with systems like B-Fabric, "ad-hoc visualizations and analytics can be realized on top of an integrative platform that captures and processes any kind of data and scientific processes." The platform is also able "to decouple metadata management from data processing and visualization components" while also optimizing specific software software environments for different research areas.
In this 2022 journal article published in Journal of Integrative Bioinformatics, Panse et al. discuss the state of research-based core facilities that share research resources across institutions or organizations, in particular in regards to data and information management. The authors note that the "interdisciplinary nature of such scientific projects requires a powerful and flexible platform for data annotation, communication, and exploration," and the authors—associated with the Functional Genomics Center Zurich (FGCZ)—present B-Fabric, their solution to data and information management in such settings. After providing a brief background, the authors discuss the development and application of B-Fabric at FGCZ, along with lessons learned. They conclude that with systems like B-Fabric, "ad-hoc visualizations and analytics can be realized on top of an integrative platform that captures and processes any kind of data and scientific processes." The platform is also able "to decouple metadata management from data processing and visualization components" while also optimizing specific software software environments for different research areas.
 Genotyping of Cannabis sativa is a useful component of not only gender identification of plants for cultivators but also other types of laboratory testing and research of Cannabis. Performing such tests in a reliable, cost-effective, and high-performance way has significant value for Cannabis testing laboratories. In this 2022 journal article, Torres et al. compare a number of different genotyping techniques, ultimately demonstrating some of that value. After providing background information and their methodology, the authors discuss the results of gender testing using a wide array of genotyping techniques. They conclude that "the multiple methodologies presented here allow for accurate, quick, and cost-effective screening that will enable future development of germplasm and the industry." They add that their "real-time assays can be performed by cannabis testing laboratories performing diagnostic testing, as well as in the field with minimal molecular biology equipment and expertise."
Genotyping of Cannabis sativa is a useful component of not only gender identification of plants for cultivators but also other types of laboratory testing and research of Cannabis. Performing such tests in a reliable, cost-effective, and high-performance way has significant value for Cannabis testing laboratories. In this 2022 journal article, Torres et al. compare a number of different genotyping techniques, ultimately demonstrating some of that value. After providing background information and their methodology, the authors discuss the results of gender testing using a wide array of genotyping techniques. They conclude that "the multiple methodologies presented here allow for accurate, quick, and cost-effective screening that will enable future development of germplasm and the industry." They add that their "real-time assays can be performed by cannabis testing laboratories performing diagnostic testing, as well as in the field with minimal molecular biology equipment and expertise."
 In this 2022 journal article published in Applied Sciences, Mustapää et al. turn to the calibration process of the pharmaceutical industry—as well as their regulatory- and standards-based requirements—as inspiration for building a proof-of-concept software platform that better ensures "the preservation of traceability and data integrity" of calibration data. After a lengthy and thorough background to the topic of laboratory calibrations and calibration data management, the authors present their proof of concept software platform, designed "to harmonize the data exchange between the parties of a calibration chain to enable automation of the data management processes in the receiving end." They then discuss the results of their efforts, challenges in advancing such metrology infrastructure, and topics that require further research. They conclude that the real use cases tested on their proof of concept "proved that the multitenant platform is an efficient means of organizing the exchange of [digital calibration certificates]," and that "the system would suit the requirements of other industries as well, providing cost-efficiency through economies of scale."
In this 2022 journal article published in Applied Sciences, Mustapää et al. turn to the calibration process of the pharmaceutical industry—as well as their regulatory- and standards-based requirements—as inspiration for building a proof-of-concept software platform that better ensures "the preservation of traceability and data integrity" of calibration data. After a lengthy and thorough background to the topic of laboratory calibrations and calibration data management, the authors present their proof of concept software platform, designed "to harmonize the data exchange between the parties of a calibration chain to enable automation of the data management processes in the receiving end." They then discuss the results of their efforts, challenges in advancing such metrology infrastructure, and topics that require further research. They conclude that the real use cases tested on their proof of concept "proved that the multitenant platform is an efficient means of organizing the exchange of [digital calibration certificates]," and that "the system would suit the requirements of other industries as well, providing cost-efficiency through economies of scale."
















