January 5, 2021 - How Wastewater Labs Can Help Defeat the Pandemic

Health officials are turning to wastewater systems to track the spread of COVID-19 and identify vulnerable communities. Clinical labs that perform coronavirus testing do not understand the methodologies for wastewater analysis. As the National Wastewater Surveillance System comes online, wastewater testing labs can take on a critical role in bringing the pandemic under control...
[Read More]
January 5, 2021 - Strategies for Creating Effective Technical Documentation – Part 3
In part 2 of our blog series on creating effective technical documentation, we detailed a best practice methodology that ensures the documentation will maximize benefits for your organization (part 1).January 5, 2021 - Does Your LIMS Need a Tune Up?
 It might now be several years since you first deployed your LIMS, but have you paid it the attention it deserves? Your laboratory could be running much greater sample numbers or your lab processes and workflows may have changed over time. It could be time to think about a ‘tune up’.
It might now be several years since you first deployed your LIMS, but have you paid it the attention it deserves? Your laboratory could be running much greater sample numbers or your lab processes and workflows may have changed over time. It could be time to think about a ‘tune up’.
Surprisingly, small configuration changes can make a big difference to the efficiency of your laboratory operation. [Read More]
December 30, 2020 - Lab Informatics Insights 2020
 It’s typical for a service provider to stay up-to-date with industry happenings and to continually learn about their potential clients’ ever-changing needs. There are programs and measures in place to calculate and monitor these needs. At CSols, we developed a set of polls and quizzes to identify industry trends and collect feedback from those whom our lab informatics services can help. The respondents were contacts across various industries, and at different stages in their lab informatics project. [Read More]
It’s typical for a service provider to stay up-to-date with industry happenings and to continually learn about their potential clients’ ever-changing needs. There are programs and measures in place to calculate and monitor these needs. At CSols, we developed a set of polls and quizzes to identify industry trends and collect feedback from those whom our lab informatics services can help. The respondents were contacts across various industries, and at different stages in their lab informatics project. [Read More]
December 30, 2020 - Diversification: The Key to Surviving as a Cannabis Testing Lab

Organizations, Businesses, Schools and Colleges everywhere need testing programs. Data-driven decision making has put laboratories squarely in the center of the COVID-19 pandemic response. Unfortunately, even CLIA-certified clinical diagnostic labs can’t contribute unless they have the resources to conduct COVID-19 molecular diagnostics testing. But now there may...
[Read More]
December 30, 2020 - Laboratory Informatics Webcast Series – The Digital Laboratory: What the Future Holds
Astrix Technology Group has been helping scientific organizations implement and integrate new informatics systems in the laboratory since 1995. Our experienced team of expert informatics consultants bring together technical, strategic, regulatory and content knowledge to provide the most effective solutions to problems faced by scientific organizations. Astrix partners with many of the industry leaders in this space and have brought them together for a one of kind webcast series that will delve into the future of lab informatics and technology. [Read More]December 30, 2020 - Positioning the Health System to Weather the Storm of COVID-19
 Five areas of focus emerge when we look at how best-of-breed technology is helping labs manage through COVID-19. Get the new white paper. [READ MORE]
Five areas of focus emerge when we look at how best-of-breed technology is helping labs manage through COVID-19. Get the new white paper. [READ MORE]
December 30, 2020 - Cloud Hosting – Makes Sense
 In recent years, cloud technology has evolved into a universal essential technology platform, transforming the healthcare industry as we know it. Introducing the cloud-based laboratory information system, which is expected to become the leading trend in the laboratory information systems market. This advancement provides storage and servers over the internet at a reduced cost, as well as increasing speed, productivity, and security of the systems in the network.
SCC Soft Computer’s diverse product solutions are all available on the cloud, offering unique features and benefits per module. Click here to learn more about our products hosted on the cloud.
In recent years, cloud technology has evolved into a universal essential technology platform, transforming the healthcare industry as we know it. Introducing the cloud-based laboratory information system, which is expected to become the leading trend in the laboratory information systems market. This advancement provides storage and servers over the internet at a reduced cost, as well as increasing speed, productivity, and security of the systems in the network.
SCC Soft Computer’s diverse product solutions are all available on the cloud, offering unique features and benefits per module. Click here to learn more about our products hosted on the cloud.
December 30, 2020 - Managing Cannabis Information Workflows with LIMS
 Since March 2017 doctors in Germany can prescribe cannabis based drugs for several illnesses and conditions such as headaches or migraines. Legalizing medical cannabis is paving the way for many smaller biotech companies and startups in Germany in businesses related to cannabis research, cannabis cultivation, or manufacturing cannabis-based products. These new regulations have led to the emergence of a number of cannabis-testing laboratories and massive facilities for growing and manufacturing medical-grade cannabis.[Read More]
Since March 2017 doctors in Germany can prescribe cannabis based drugs for several illnesses and conditions such as headaches or migraines. Legalizing medical cannabis is paving the way for many smaller biotech companies and startups in Germany in businesses related to cannabis research, cannabis cultivation, or manufacturing cannabis-based products. These new regulations have led to the emergence of a number of cannabis-testing laboratories and massive facilities for growing and manufacturing medical-grade cannabis.[Read More]
December 22, 2020 - LIMS Validation – Get Yourself Armed with Knowledge This Festive Season
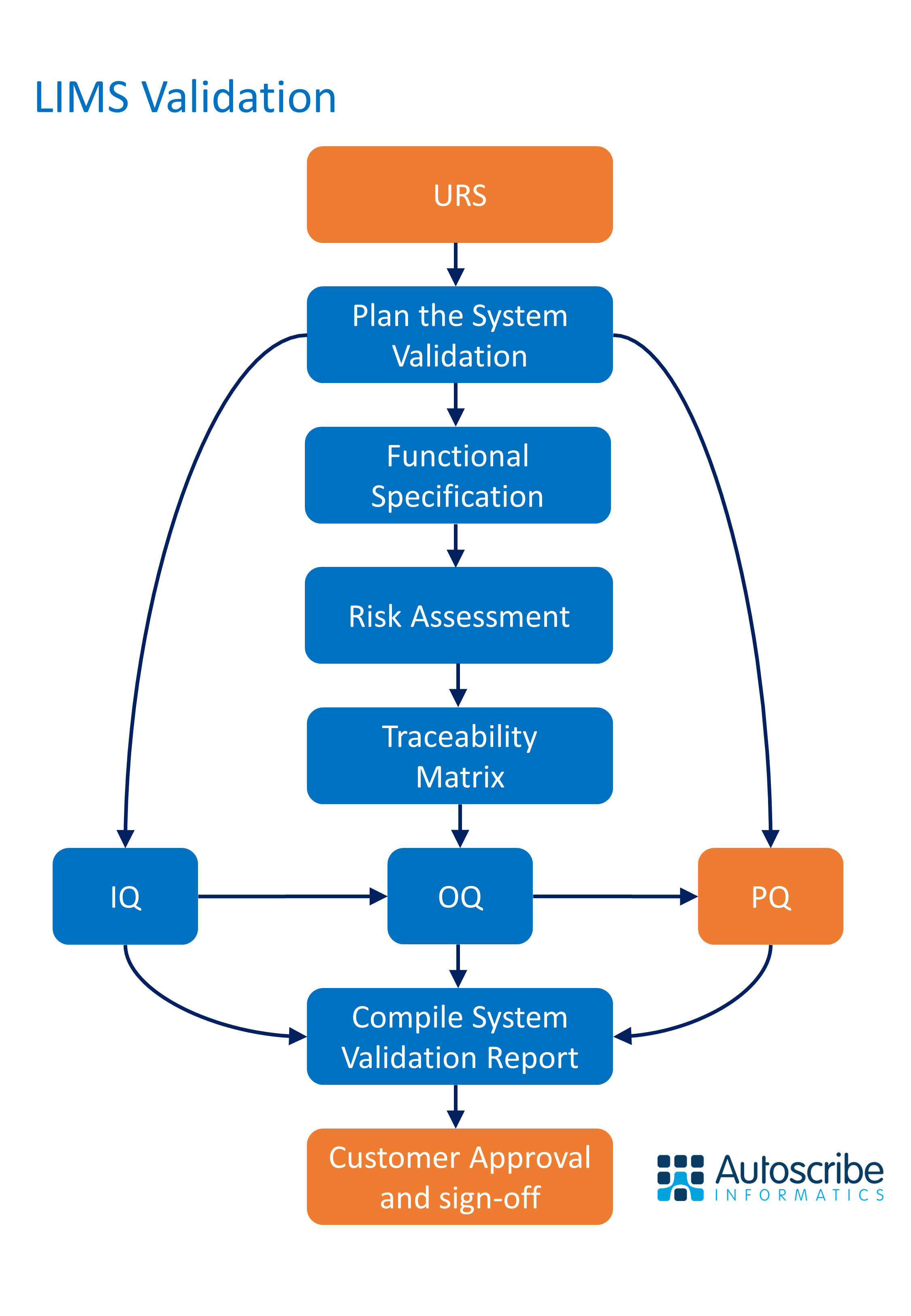 LIMS Validation can be a tricky subject to get your head round. This white paper provides a good background on what LIMS validation is, and the steps you need to go through to achieve it. Whatever LIMS software you are using the steps and the jargon are the same.
LIMS Validation can be a tricky subject to get your head round. This white paper provides a good background on what LIMS validation is, and the steps you need to go through to achieve it. Whatever LIMS software you are using the steps and the jargon are the same.
Grab a copy today and read it at your leisure:
[Download White Paper from Here]
Wishing you a happy festive season,
From Autoscribe Informatics and all in the LIMS world!
December 15, 2020 - How to Conduct Effective User Acceptance Testing
 According to a report from Forbes, 25 percent of technology projects fail outright; 20 to 25 percent don’t show any return on investment; and as much as 50 percent need massive reworking by the time they’re finished.
Why is this the case? It is a complex question that likely comes with complex answers. Technical failures, scope creep, platform and performance issues, and the like all happen rampantly in enterprise IT organizations. [Read More]
According to a report from Forbes, 25 percent of technology projects fail outright; 20 to 25 percent don’t show any return on investment; and as much as 50 percent need massive reworking by the time they’re finished.
Why is this the case? It is a complex question that likely comes with complex answers. Technical failures, scope creep, platform and performance issues, and the like all happen rampantly in enterprise IT organizations. [Read More]
December 15, 2020 - How Labs Can Get Involved in COVID Screening Programs

Organizations, Businesses, Schools and Colleges everywhere need testing programs. Data-driven decision making has put laboratories squarely in the center of the COVID-19 pandemic response. Unfortunately, even CLIA-certified clinical diagnostic labs can’t contribute unless they have the resources to conduct COVID-19 molecular diagnostics testing. But now there may...
[Read More]
December 15, 2020 - Sunquest provides NGS Support from the Wet Bench to the Dry Lab
 Molecular labs conducting next-generation sequencing (NGS) must analyze troves of data to interpret results. In addition to Sunquest Mitogen LIMS, Sunquest also offers Sunquest Mitogen Genetics. This powerful genomic analysis support software to helps labs more easily classify genetic variants and quickly produce clinically-actionable patient reports.
[Read More]
Molecular labs conducting next-generation sequencing (NGS) must analyze troves of data to interpret results. In addition to Sunquest Mitogen LIMS, Sunquest also offers Sunquest Mitogen Genetics. This powerful genomic analysis support software to helps labs more easily classify genetic variants and quickly produce clinically-actionable patient reports.
[Read More]
December 15, 2020 - CSols University – SampleManager eLearning Courses
Promo code for December: GIFT40 to get 40% any or all three courses! CSols University has a series of three online courses that can help. In just a few short hours you can go from beginner to report and workflow guru, for 40% off in the month of December with coupon code: GIFT40. Gift yourself this month with in-depth SampleManager LIMS knowledge.December 8, 2020 - Data Visualization: Make the Most of Your Lab Informatics
 You’ve probably seen the buzzwords data visualization, big data, and machine learning if you’ve been reading a lot in the last year or so. And who hasn’t been reading a lot during 2020—there’s not that much else to do! In this blog post, we’ll discuss some of the ways that data visualization tools can help you use accumulated data (even if it’s big data)... [Read More]
You’ve probably seen the buzzwords data visualization, big data, and machine learning if you’ve been reading a lot in the last year or so. And who hasn’t been reading a lot during 2020—there’s not that much else to do! In this blog post, we’ll discuss some of the ways that data visualization tools can help you use accumulated data (even if it’s big data)... [Read More]
December 8, 2020 - How Your LIMS Can Help You Deliver Top Notch Customer Service: Part 2
In Part 1 of this blog post, we shared two options your lab has for using your LIMS to promote exceptional customer service: EMR integration and a provider portal. We also explored a few features your portal must have to be effective. Here, we’ll continue looking at the provider portal, focusing on specific functionality your lab should consider when partnering with a LIMS vendor for this technology. [Read More]December 8, 2020 - Astrix Case Study: Waters Empower Custom Fields Development and Validation
 Overview
Overview
This project involved one of the world’s fastest-growing pharmaceutical companies with an established commercial presence in over 100 different countries and annual revenue of around 15 billion dollars. The company has a wide-ranging portfolio of both name brand and generic therapeutic products that treat many different disease conditions, and also produces medical aesthetics. [Read More]
December 8, 2020 - The COVID Testing Catch-22

The key to managing the pandemic, and why we‘ll never do it perfectly. Everybody agrees the only way to manage the SARS-CoV-2 pandemic is to know how bad it is by conducting tests, and lots of them, right? Well of course. How else? But there’s a problem. And there doesn’t seem to be a good solution. There are three types of diagnostic tests (well, really two, one is more of a...
[Read More]
December 8, 2020 - Sunquest New White Paper: Positioning the Health System to Weather the Storm of COVID-19
 Five areas of focus emerge when we look at how best-of-breed technology is helping labs manage through COVID-19. Get the new white paper.
[Read More]
Five areas of focus emerge when we look at how best-of-breed technology is helping labs manage through COVID-19. Get the new white paper.
[Read More]
December 2, 2020 - Vet Lab Blends Clinical Diagnostics with State-of-the-Art Technology Using Matrix Gemini LIMS
 In this new case study from Autoscribe Informatics BioTe Veterinary Laboratories blends clinical diagnostics with state-of-the-art technology using Matrix Gemini LIMS.
In this new case study from Autoscribe Informatics BioTe Veterinary Laboratories blends clinical diagnostics with state-of-the-art technology using Matrix Gemini LIMS.
BioTe Veterinary Laboratories is using Matrix Gemini Veterinary LIMS to manage their laboratory workflow and analytical data to drive efficient operations in their new purpose-built laboratory facility.

















