Posted on June 9, 2020
By LabLynx
Journal articles
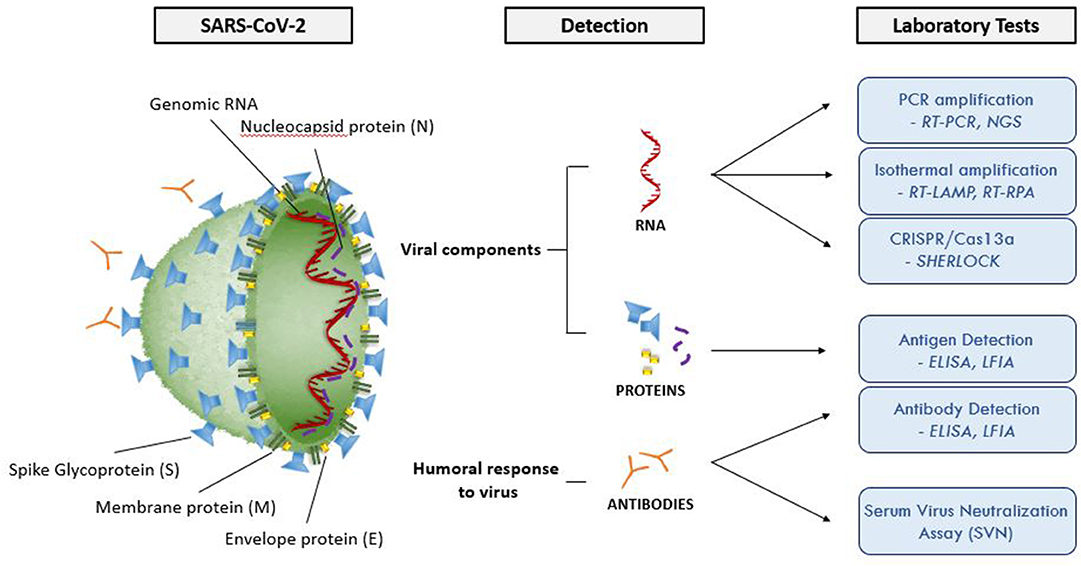
In this 2020 article published in
Frontiers in Cell and Developmental Biology, D'Cruz
et al. provide an overview of the current laboratory methods available to test for coronaviruses, with a focus on SARS-CoV-2, the virus responsible for COVID-19. After providing an introduction to COVID-19 and its virus, the authors discuss the most common methods—such as qRT-PCR, ELISA, and LFI—as well as emerging diagnostic methods involving isothermal nucleic acid amplification, CRISPR, and NGS. They conclude with several visuals comparing the methods and when they are used, and they emphasize the importance of these test methods (as well as laboratory preparedness) in addressing rapidly evolving viral infection scenarios.
Posted on June 2, 2020
By LabLynx
Journal articles
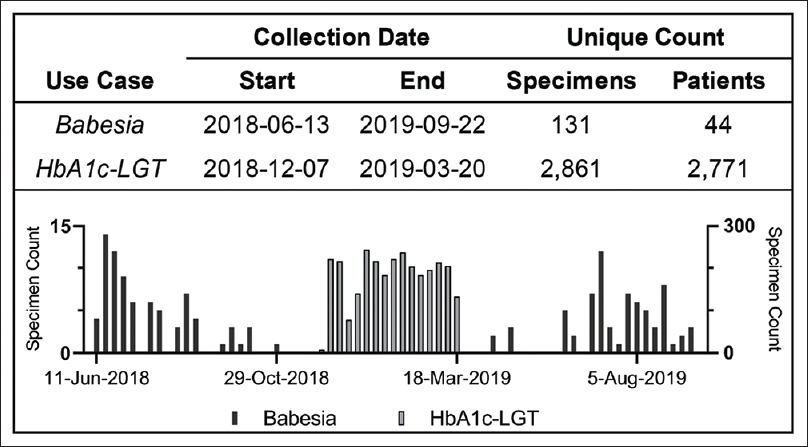
In this 2020 article published in
Journal of Pathology Informatics, Durant
et al. of the Yale New Haven Health system present the results of their attempt to automate the identification and notification of laboratory biospecimens for biomedical researchers. Noting biospecimens' value to basic, translational, and clinical research, the authors wanted to get around the "technical, logistic, regulatory, and ethical challenges" of accessing biospecimen data. They developed Prism, a tool built on open-source technology for efficiently identifying and notifying investigators of biospecimen availability in real time. The authors present details of two use cases and conclude that their "solution is highly scalable to meet the needs of even large academic centers and reference laboratories," while also guaranteeing that the virtualization of the associated workflow "within a microservices environment does not introduce a performance penalty."
Posted on May 26, 2020
By LabLynx
Journal articles
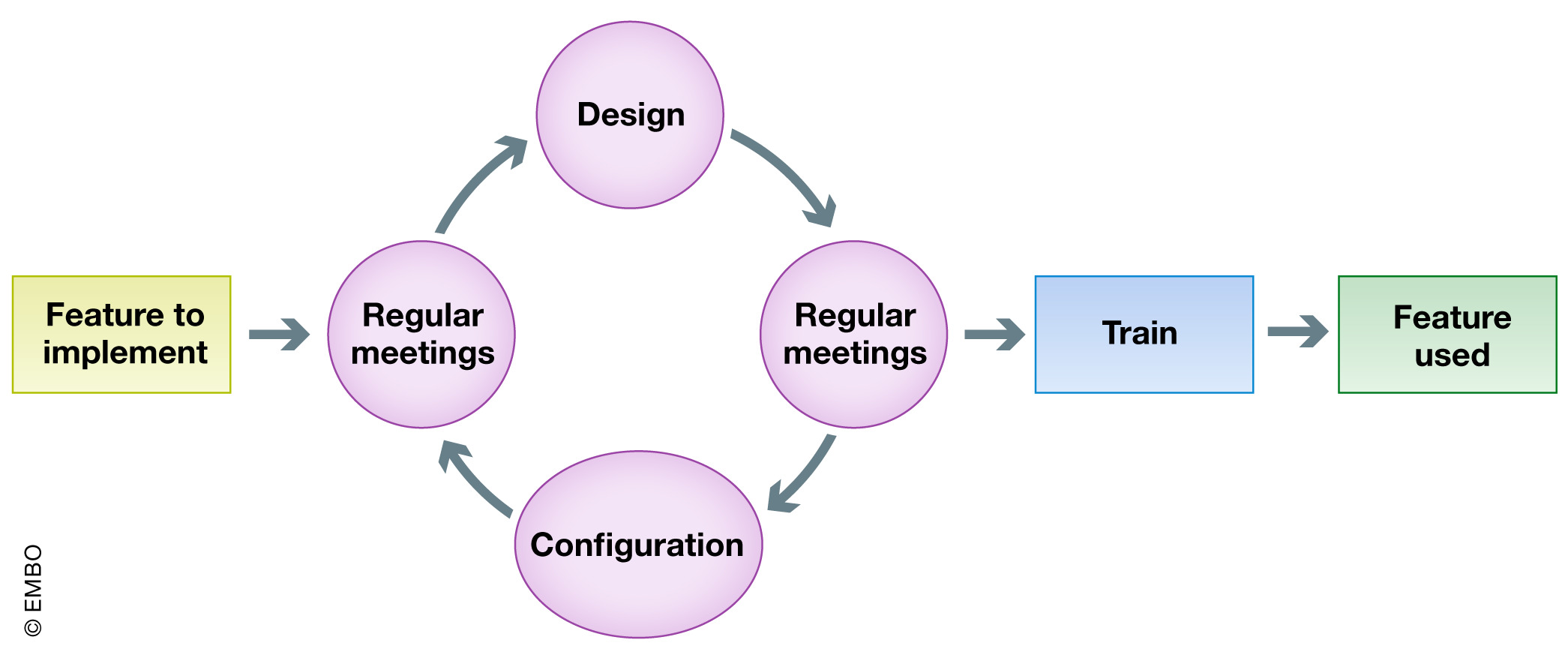
In this 2020 journal article published in
EMBO Reports, Nicolas Argento of École Polytechnique Fédérale de Lausanne (EPFL) reviews the step-by-step approach EPFL took in identifying, piloting, configuring, and implementing a combination electronic laboratory notebook (ELN)–laboratory information management system (LIMS) for member laboratories affiliated with the institution. In particular, Argento highlights the value of "surrounding yourself with the right people and the right software at the right time" when going through the various deployment steps. After providing an introduction, the author goes through the various five phases of the project they undertook, while also highlighting the value of stakeholders and other critical staff. Argento closes with a brief highlight of the "laboratory data manager" as being a role that should not be overlooked, then he concludes that while the EPFL's institutional ELN-LIMS platform meets its goals, additional data management and system adoption challenges remain.
Posted on May 18, 2020
By Shawn Douglas
Journal articles
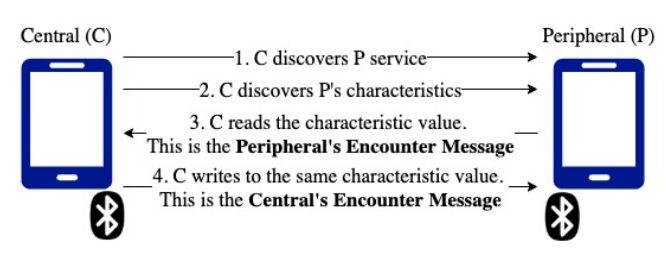
This April 2020 whitepaper authored by Bay
et al. of Singapore's Government Technology Agency presents BlueTrace, a privacy-preserving protocol that underpins Singapore's nationally deployed Bluetooth-based contact tracing system TraceTogether. The white paper describes the purpose of BlueTrace as addressing the key limitation of manual contact tracing: "an infected person can only report contacts they are acquainted with and remember having met." They describe the protocol's design considerations and implementation challenges, as well as how it meets interoperability requirements of health authorities considering its use. Finally they address potential security concerns surrounding the tech. They conclude that while not a stand-alone solution to solving contract tracing issues during a pandemic, Bluetooth-based automated tracing instances can still "ultimately coexist and support the pandemic response plans and processes of the public health authorities guiding us through" a pandemic. The authors also make available OpenTrace, an open-source reference implementation of BlueTrace, on GitHub.
Posted on May 12, 2020
By Shawn Douglas
Journal articles
In this 2019 paper published in
AIMS Public Health, Bhattacharya
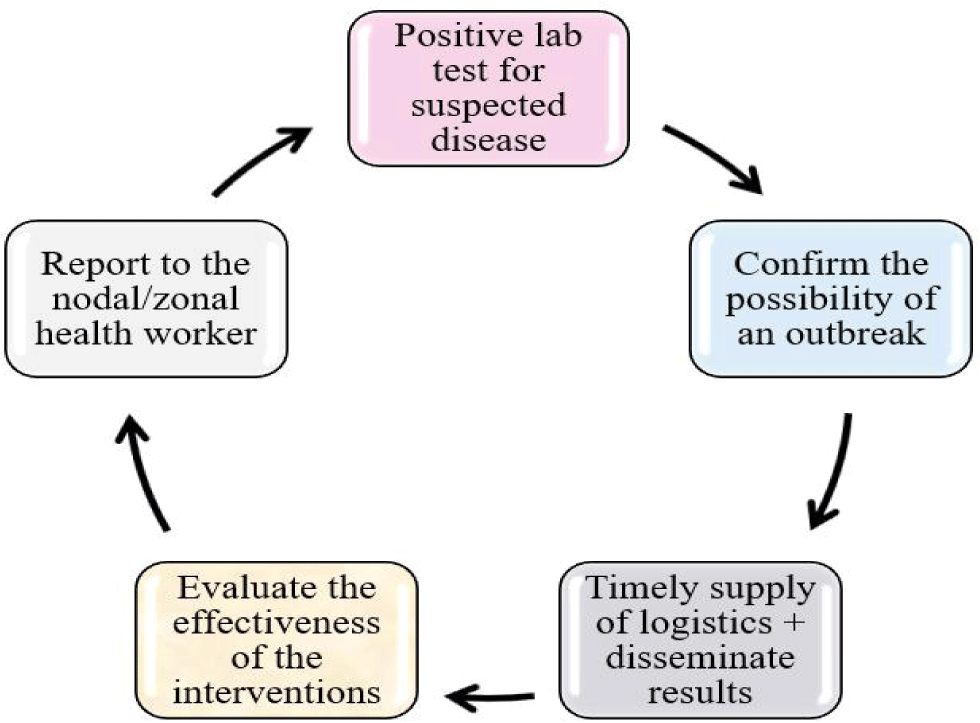
et al. argue for the benefits of using blockchain technology in public health surveillance. Noting the transparency and trust aspects of the technology, as well as the benefits of " data security and privacy, cost-effectiveness, and verifiability of data," the authors discuss four primary ways blockchain can be applied to healthcare activities, including community disease surveillance. "Blockchain could help independent organizations to manage data more efficiently during pandemics," they note, particularly in unison with machine learning and AI techniques. They conclude that blockchain-based disease surveillance "can be more effective and rapid than traditional surveillance in terms of coverage, durability, consensus, selective privacy, uniqueness, and timing."
Posted on May 5, 2020
By Shawn Douglas
Journal articles
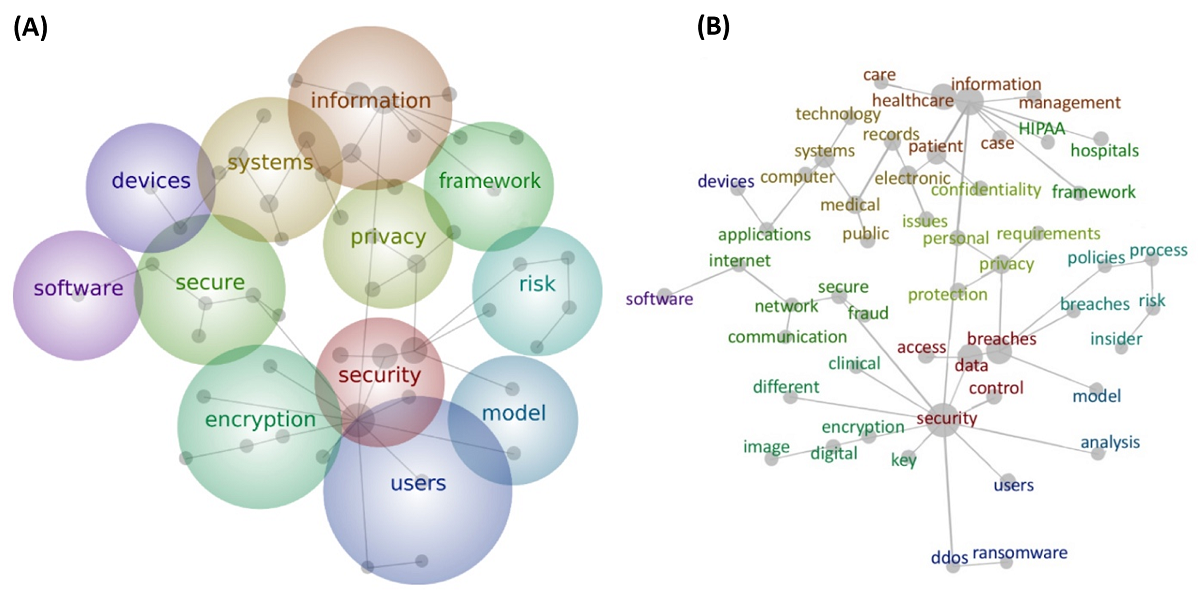
Cybersecurity remains a vital consideration for entities within the healthcare setting, with academic researchers publishing on the topic frequently. But since computer security in healthcare first started getting researched in the mid-1980s, how have topical focuses shifted? What areas, if any, are underserved by current research? Jalali
et al. attempt to answer those and other questions in this 2019 paper published in
Journal of Medical Internet Research. Using bibliometric analysis as their tool, the authors conducted chronological analysis, domain clustering analysis, and text analysis on 472 English-language articles ranging from 1985 to September 2017. The conclude that "[d]espite the increase in research and attention to cybersecurity, there are persistent shortcomings in the research on cybersecurity," including implications that emerging cyber threats, physical security methodologies, and several human elements of cybersecurity have not been sufficiently captured in research. They worry that "a school of thought that knowledge, especially specific strategies and tactics, should not be shared openly," further suppresses research growth and utility.
Posted on April 27, 2020
By Shawn Douglas
Journal articles

This week we turn back the clock a couple of years to 2018, when Fairchild
et al.published in the journal
Frontiers in Public Health their analysis of the challenges of epidemiological data reporting. With the march of COVID-19 today, their analysis and advice holds even more relevant today. In their paper, Fairchild
et al. first introduced the state of epidemiological data reporting, particularly on the internet. They then discussed the three challenges that affect such reporting: user interface, data format, and data reporting issues. They concluded with nine clear best practices that should be followed when managing epidemiological data for release to the public. They imagined a scenario of a standardized platform adopted worldwide, "where global data could be easily collected without the challenges we currently face. This would in turn streamline epidemiological and public health analysis, modeling, and informatics, resulting in better public health decision-making capabilities."
Posted on April 20, 2020
By Shawn Douglas
Journal articles
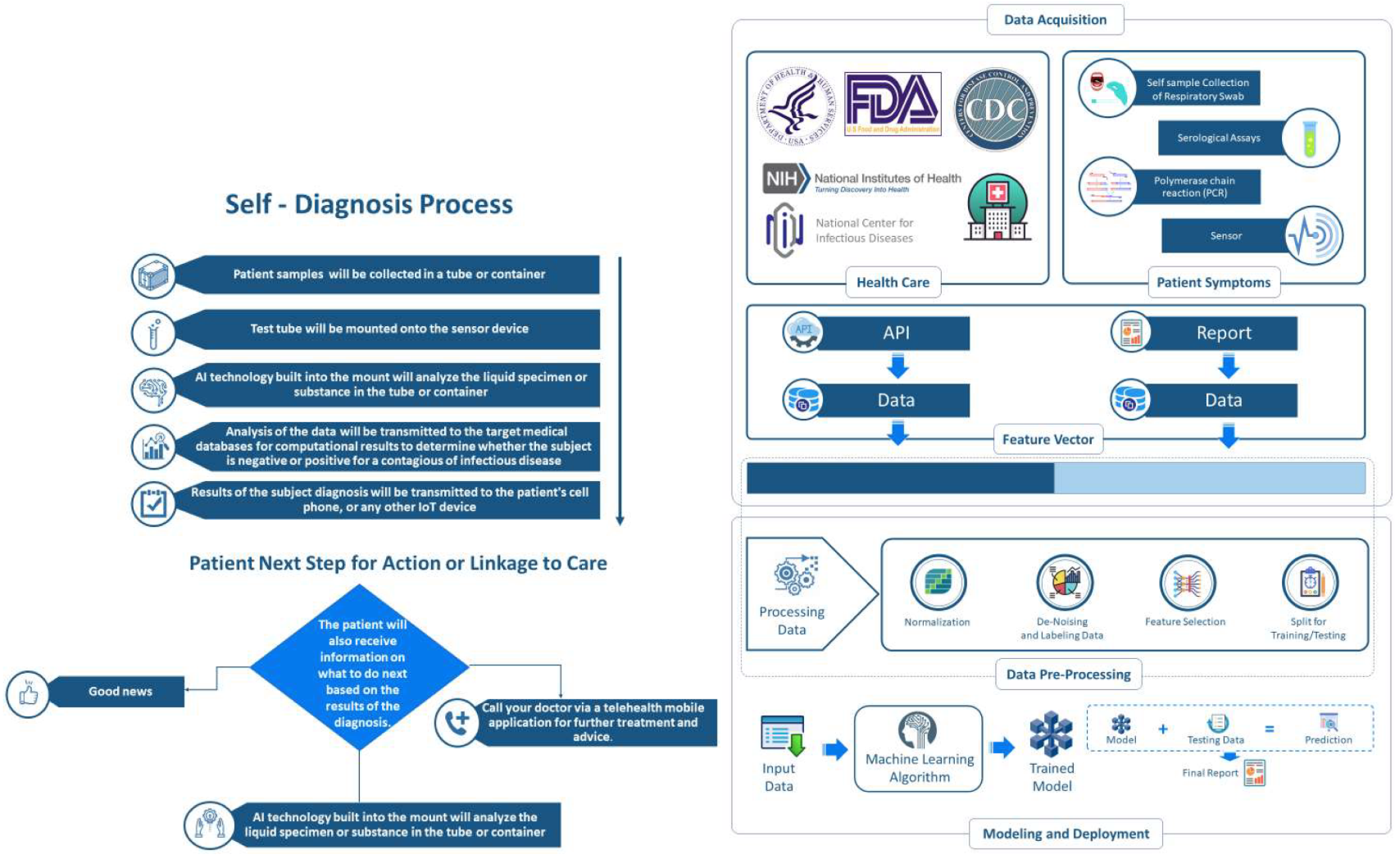
In this brief editorial in the journal
Diagnostics, Mashamba-Thompson
et al. state their case for a potentially ideal solution to COVID-19 testing in resource-poor communities: using mobile health (mHealth) solutions in conjunction with a blockchain and AI-driven data acquisition and transfer system. They add that the "AI component of this technology enables powerful data collection (patient information, geographic location of the patient, and test results), security, analysis, and curation of disparate and clinical data sets from federated blockchain platforms to derive triangulated data at very high degrees of confidence and speed. " Unfortunately, what isn't clear in their argument is how the self-testing step would actually work, let alone how the system would realistically be implemented in resource-poor settings. One could argue, however, despite the relatively few details, the sharing of ideas on how to address COVID-19 testing in various environments still has value.
Posted on April 13, 2020
By Shawn Douglas
Journal articles
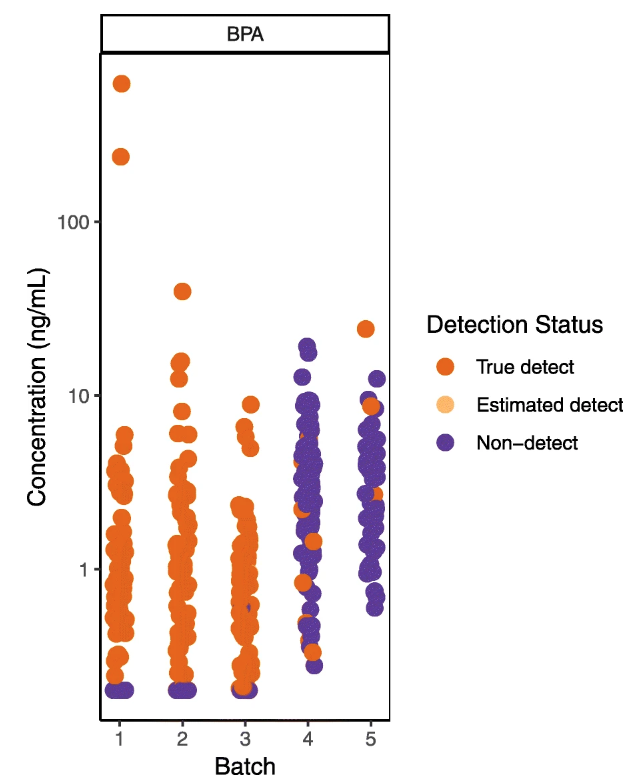
In this 2019 paper published in
Environmental Health, Udesky
et al. share their organization's guidance towards addressing quality in environmental exposure testing and data. In particular, the authors note the importance of reporting quality control data along with chemical measurements in published research, stating that its lacking leaves "readers uncertain about the level of confidence in the reported data." Their objective was to provide full guidance on the implementation, interpretation, and reporting of QC data. After explaining their approach to study design, study implementation, and data interpretation, the authors conclude that their guidance, visualizations, and supplementary content provide "a useful set of tools for getting the best information from valuable environmental exposure datasets, and enabling valid comparison and synthesis of exposure data across studies."
Posted on April 6, 2020
By Shawn Douglas
Journal articles
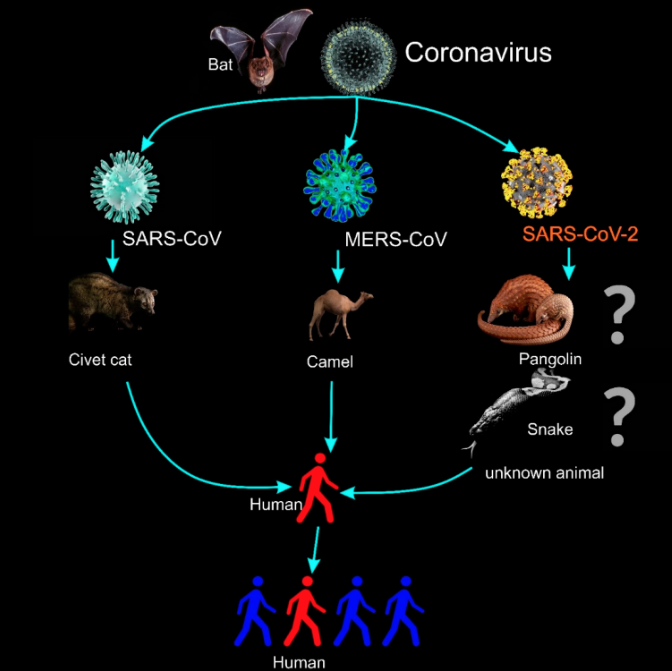
In this March 2020 journal article published in the journal
Micromachines, Nguyen, Bang, and Wolff give their professional take on point-of-care diagnostics for combating the COVID-19 epidemic. In particular, the authors discuss the loop-mediated isothermal amplification (LAMP) nucleic acid amplification technique and its potential to make COVID-19 testing faster, more portable, more flexible, more stable, and more sensitive. After discussing the then-recent events (information is changing rapidly), the authors compare real-time reverse transcription polymerase chain reaction (rRT-PCR) with LAMP methods and discuss other important factors affecting society because of the pandemic.
Posted on March 30, 2020
By Shawn Douglas
Journal articles
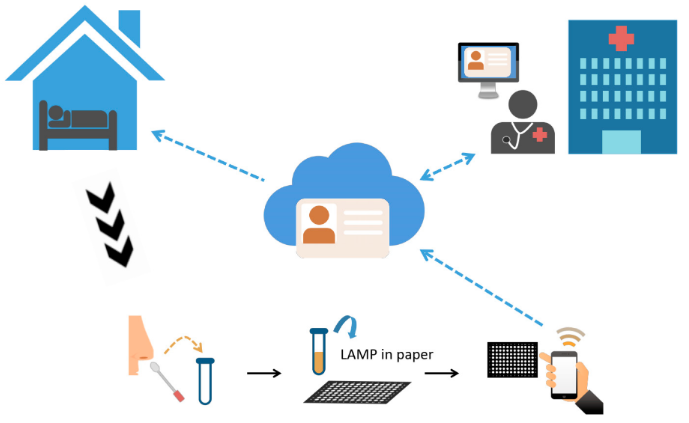
In this brief editorial article published in the journal
Diagnostics, Tiawanese researchers Yang
et al. provide their insights into a more convenient at-home point-of-care (POC) testing method for the COVID-19 illness. Noting the urgency of the associated pandemic and the various costs associated with traditional testing methodologies such as lateral flow immunoassays and molecular-based assays, the authors suggest the blending of several tools: a paper-based result and common mobile devices. A patient at home could take a nasal swab and use a POC device to return a colorimetric result, which could then be captured by mobile phone and rapidly sent to a clinician for analysis. They conclude that such a method could "provide new insights into designing POC COVID-19 diagnostics and ultimately improve the health care system to combat this and similar diseases."
Posted on March 24, 2020
By Shawn Douglas
Journal articles
This is the third of a series of interim guidance documents, this one originating from the U.S. Food and Drug Administration (FDA). Issued on March 16, this guidance document provides insight into FDA policy for the development, validation, and use of diagnostic tests for Coronavirus Disease-2019 (COVID-19), particularly those tests issued an Emergency Use Authorization (EUA). This guidance applies to not only manufacturers developing and distributing test kits but also CLIA laboratories cleared for high-complexity testing that develop and validate their own tests. The FDA provides background on the topic and then discusses the policy in detail, before closing with its validation study recommendations.
Posted on March 24, 2020
By Shawn Douglas
Journal articles
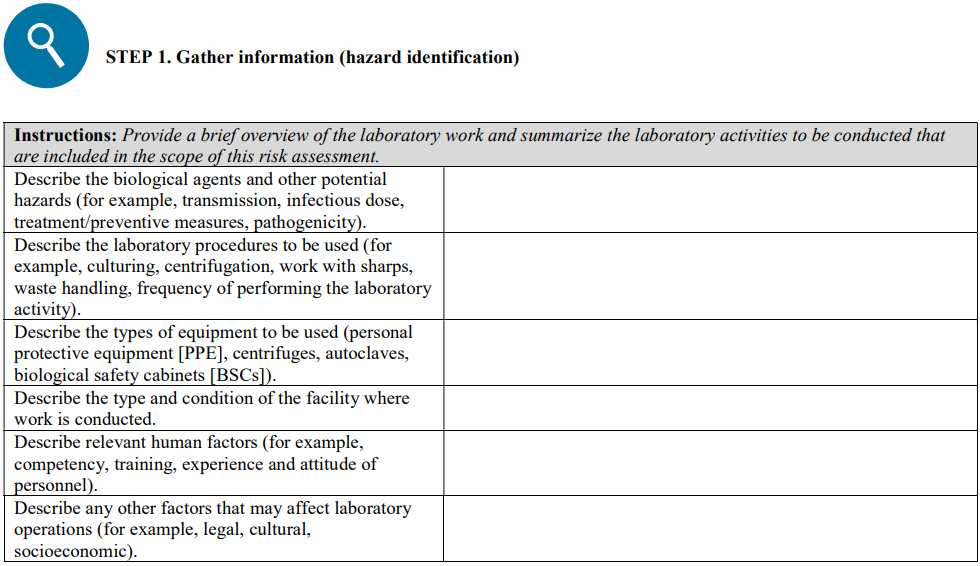
Again, not a journal article, but here rather we have another interim guidance document by the World Health Organization (WHO), this time in regards to laboratory biosafety related to COVID-19 testing. Effective March 19, 2020, this guidance document, complete with references, addresses laboratory biosafety principles to consider when handling and testing specimens believed to contain the COVID-19 virus. The WHO provides a bit of background, provides an overview of the key points, and then discusses those points in further detail. They also include two annex items: the core requirements of good microbiological practice and procedure (GMPP) and a risk assessment template for laboratories to borrow from.
Posted on March 22, 2020
By Shawn Douglas
Journal articles
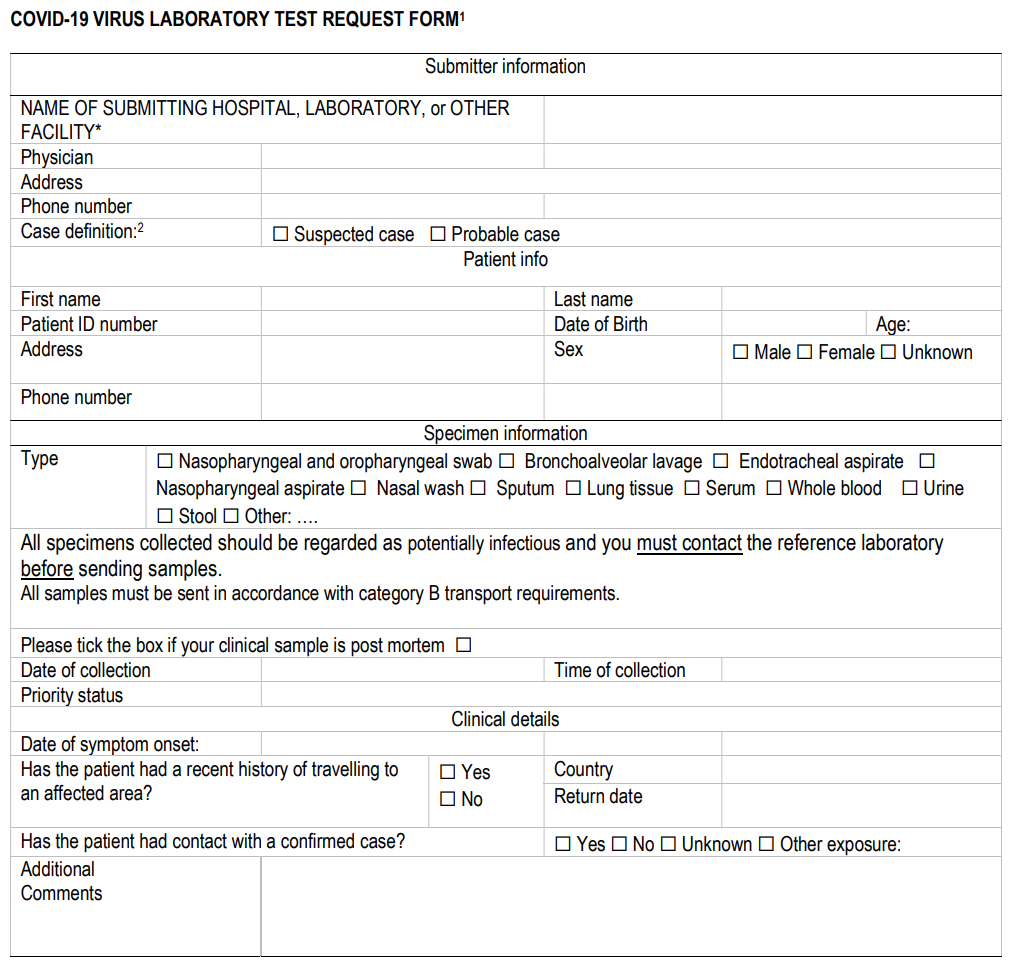
Breaking from the norm, rather than a journal article, this document represents official interim guidance on laboratory testing for COVID-19 from the World Health Organization (WHO), effective March 19, 2020. This guidance document, complete with references, addresses laboratory testing guidance principles for testing patients who meet the case definition for being infected with the COVID-19 virus. The WHO provides a bit of background, then addresses specimen collection and shipment, laboratory testing procedures, and reporting. They also include where further research must tread, as well as an ISO 15189:2012-compliant COVID-19 laboratory test request form. WHO closes by encouraging "the sharing of data to better understand and thus manage the COVID-19 outbreak, and to develop countermeasures."
Posted on March 16, 2020
By Shawn Douglas
Journal articles

In this 2019 journal article published in the
African Journal of Laboratory Medicine, Mtonga
et al. describe the efforts of developing a laboratory information system (LIS) for resource-poor regions, as well the benefits of other informatics interventions in those settings. Using open-source C4G BLIS as their base, the researchers then turned to "user stories" as a recommended method for determining the system requirements the end users required, then updating with those requirements as well as critical functionality that would suitable for resource-poor hospitals and labs. The authors then deployed the resulting LIS in Kamuzu Central Hospital in Lilongwe, Malawi. They conclude their experience "highlights the potential of using informatics interventions to address systemic problems in the laboratory testing process in low-resource settings," though the implementation of those interventions "may require innovation of new hardware to address various contextual issues."
Posted on March 9, 2020
By Shawn Douglas
Journal articles
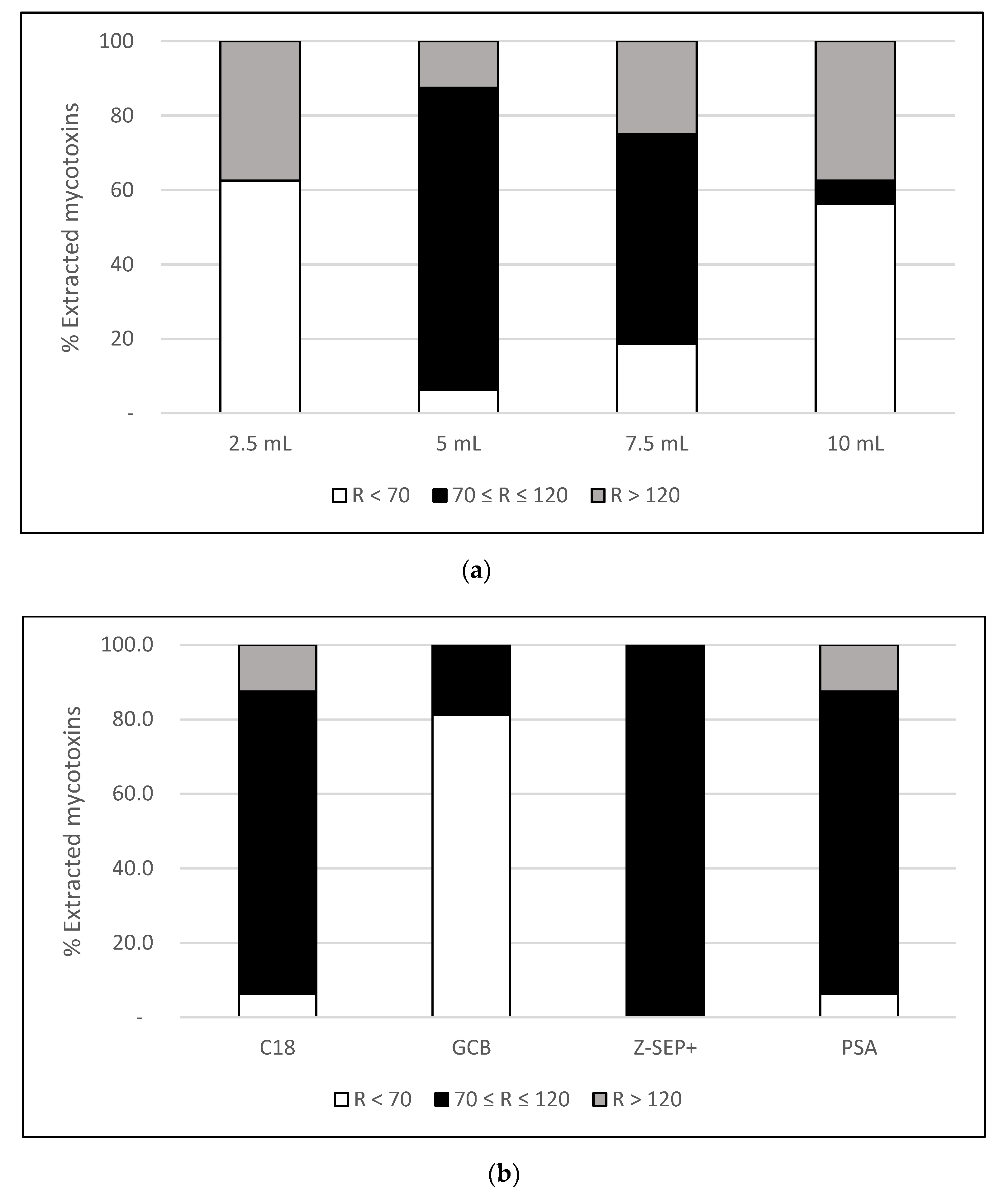
In this 2020 paper published in the journal
Toxins, Narváez
et al. of Spain and Italy present the results of their attempt to use a specific type of chromatography and spectrometry in order to analyze the amount of mycotoxins and pesticides in nutraceuticals made from
Cannabis sativa and other botanicals. The authors developed specific procedures associated with a "methodology using ultra-high-performance liquid chromatography coupled with quadrupole-Orbitrap high-resolution mass spectrometry (UHPLC-Q-Orbitrap HRMS)." After testing their methodology on 10 cannabidiol (CBD) supplements, they found numerous mycotoxins and dozens of pesticides. They conclude that their "results highlight the necessity of monitoring contaminants in food supplements in order to ensure safe consumption" while also demonstrating the effectiveness of their methodology in doing so.
Posted on March 2, 2020
By Shawn Douglas
Journal articles
In this 2019 paper published in
Journal of Medical Biochemistry, Lippi and Mattiuzi examine the primary drivers and outcomes of implementing project management strategies in the field of laboratory medicine. Citing funding and personnel shortages in healthcare services, as well as an increasing volume and complexity of analytical tests in the lab, the authors point out the importance of proper project management in the various aspects of the clinical laboratory and its organization and operation in order to better ensure more rapid and accurate diagnoses and improved clinical outcomes. They lay out four primary steps and address additional drivers to consider when implementing project management into laboratory planning and operations, including defining the laboratory environment, planning technical resources, managing staff, and interacting with hospital administrators and other important stakeholders. They conclude that through these activities, as well as some final last steps, clinical laboratories—and by extension the patients they serve—can benefit greatly from incorporating thoughtful project planning into their activities.
Posted on February 24, 2020
By Shawn Douglas
Journal articles
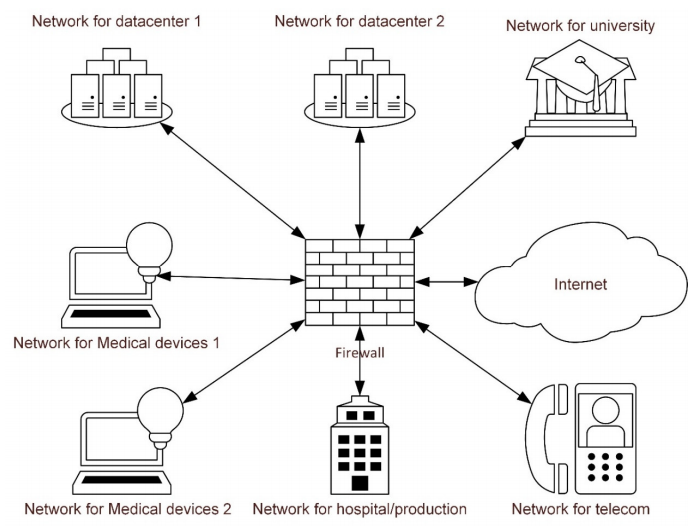
Cybersecurity is an increasingly relevant and critical topic for most industries, including the healthcare industry. In hospitals and other clinical settings, patient monitoring systems, medical imaging systems, and laboratory instruments are increasingly networked to get the most out of data management and operational efficiency. However, these devices are at risk of cyber attack, with potentially significant consequences. Johansson
et al. acknowledge this problem and attempt to address the perceptions of information and medical technology experts on the topic of securing in-house medical devices through the practice of network segmentation. The authors present their discussion results with 18 stakeholders from the Swedish Region Skåne, and they conclude that the stakeholders "were positively receptive to the increase in cybersecurity provided by network segmentation but concerned about the increase in the administration that it will entail for medical devices." They note that these and other opinions stated in the research are vital for health networks to take into account when considering improving their cybersecurity.
Posted on February 17, 2020
By Shawn Douglas
Journal articles
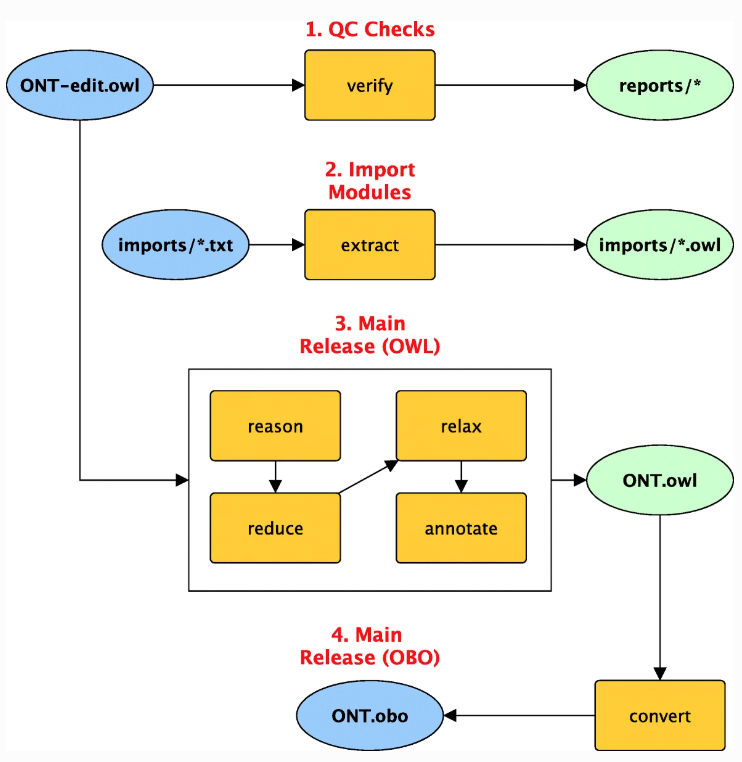
Ontologies are important to biological and biomedical researchers working with multiple data sources because, when implemented well, ontologies ensure heterogeneous, multimodal data across multiple domains and they aid with more effective data mining and discoveries. Ontologies also assist in improving data quality. In this 2019 paper, Jackson
et al. highlight the importance of ontologies with their ROBOT open-source library, which aids with the automation of ontology development. They describe the various means which ROBOT "provides ontology processing commands for a variety of tasks, including commands for converting formats, running a reasoner, creating import modules, running reports, and various other tasks." They conclude that such automation allows developers to easily "configure, combine, and execute individual tasks in comprehensive, automated workflows." The authors also demonstrate how ROBOT can help catch logical errors and provide quality control mechanisms to ontology creation and updating.
Posted on February 10, 2020
By Shawn Douglas
Journal articles
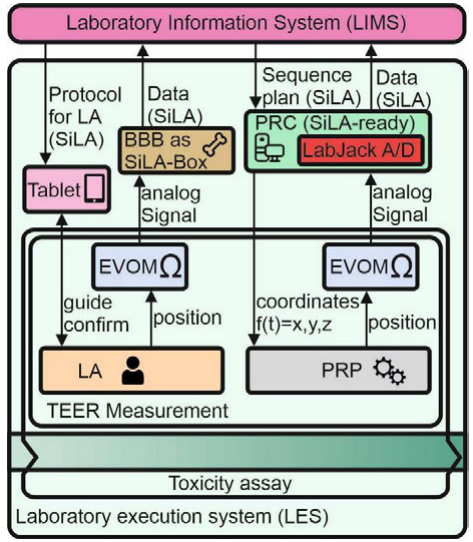
In this brief article by Schmieder
et al., the authors provide an example of applying laboratory informatics to research activities for drug discovery and personalized medicine. In particular, the authors look at the
in vitro nephrotoxicity assay and the use of transepithelial electrical resistance (TEER) to evaluate the barrier function of cellular layers. Citing issues such as the significant multi-step processes involved with this type of testing, the authors attempt to demonstrate how a laboratory information management system (LIMS), laboratory execution system (LES), and automation standards such as SiLA can be used together to decrease the workflow complexity of such cell-based assays.
 In this 2020 article published in Frontiers in Cell and Developmental Biology, D'Cruz et al. provide an overview of the current laboratory methods available to test for coronaviruses, with a focus on SARS-CoV-2, the virus responsible for COVID-19. After providing an introduction to COVID-19 and its virus, the authors discuss the most common methods—such as qRT-PCR, ELISA, and LFI—as well as emerging diagnostic methods involving isothermal nucleic acid amplification, CRISPR, and NGS. They conclude with several visuals comparing the methods and when they are used, and they emphasize the importance of these test methods (as well as laboratory preparedness) in addressing rapidly evolving viral infection scenarios.
In this 2020 article published in Frontiers in Cell and Developmental Biology, D'Cruz et al. provide an overview of the current laboratory methods available to test for coronaviruses, with a focus on SARS-CoV-2, the virus responsible for COVID-19. After providing an introduction to COVID-19 and its virus, the authors discuss the most common methods—such as qRT-PCR, ELISA, and LFI—as well as emerging diagnostic methods involving isothermal nucleic acid amplification, CRISPR, and NGS. They conclude with several visuals comparing the methods and when they are used, and they emphasize the importance of these test methods (as well as laboratory preparedness) in addressing rapidly evolving viral infection scenarios.
 In this 2020 article published in Journal of Pathology Informatics, Durant et al. of the Yale New Haven Health system present the results of their attempt to automate the identification and notification of laboratory biospecimens for biomedical researchers. Noting biospecimens' value to basic, translational, and clinical research, the authors wanted to get around the "technical, logistic, regulatory, and ethical challenges" of accessing biospecimen data. They developed Prism, a tool built on open-source technology for efficiently identifying and notifying investigators of biospecimen availability in real time. The authors present details of two use cases and conclude that their "solution is highly scalable to meet the needs of even large academic centers and reference laboratories," while also guaranteeing that the virtualization of the associated workflow "within a microservices environment does not introduce a performance penalty."
In this 2020 article published in Journal of Pathology Informatics, Durant et al. of the Yale New Haven Health system present the results of their attempt to automate the identification and notification of laboratory biospecimens for biomedical researchers. Noting biospecimens' value to basic, translational, and clinical research, the authors wanted to get around the "technical, logistic, regulatory, and ethical challenges" of accessing biospecimen data. They developed Prism, a tool built on open-source technology for efficiently identifying and notifying investigators of biospecimen availability in real time. The authors present details of two use cases and conclude that their "solution is highly scalable to meet the needs of even large academic centers and reference laboratories," while also guaranteeing that the virtualization of the associated workflow "within a microservices environment does not introduce a performance penalty."
 In this 2020 journal article published in EMBO Reports, Nicolas Argento of École Polytechnique Fédérale de Lausanne (EPFL) reviews the step-by-step approach EPFL took in identifying, piloting, configuring, and implementing a combination electronic laboratory notebook (ELN)–laboratory information management system (LIMS) for member laboratories affiliated with the institution. In particular, Argento highlights the value of "surrounding yourself with the right people and the right software at the right time" when going through the various deployment steps. After providing an introduction, the author goes through the various five phases of the project they undertook, while also highlighting the value of stakeholders and other critical staff. Argento closes with a brief highlight of the "laboratory data manager" as being a role that should not be overlooked, then he concludes that while the EPFL's institutional ELN-LIMS platform meets its goals, additional data management and system adoption challenges remain.
In this 2020 journal article published in EMBO Reports, Nicolas Argento of École Polytechnique Fédérale de Lausanne (EPFL) reviews the step-by-step approach EPFL took in identifying, piloting, configuring, and implementing a combination electronic laboratory notebook (ELN)–laboratory information management system (LIMS) for member laboratories affiliated with the institution. In particular, Argento highlights the value of "surrounding yourself with the right people and the right software at the right time" when going through the various deployment steps. After providing an introduction, the author goes through the various five phases of the project they undertook, while also highlighting the value of stakeholders and other critical staff. Argento closes with a brief highlight of the "laboratory data manager" as being a role that should not be overlooked, then he concludes that while the EPFL's institutional ELN-LIMS platform meets its goals, additional data management and system adoption challenges remain.
 This April 2020 whitepaper authored by Bay et al. of Singapore's Government Technology Agency presents BlueTrace, a privacy-preserving protocol that underpins Singapore's nationally deployed Bluetooth-based contact tracing system TraceTogether. The white paper describes the purpose of BlueTrace as addressing the key limitation of manual contact tracing: "an infected person can only report contacts they are acquainted with and remember having met." They describe the protocol's design considerations and implementation challenges, as well as how it meets interoperability requirements of health authorities considering its use. Finally they address potential security concerns surrounding the tech. They conclude that while not a stand-alone solution to solving contract tracing issues during a pandemic, Bluetooth-based automated tracing instances can still "ultimately coexist and support the pandemic response plans and processes of the public health authorities guiding us through" a pandemic. The authors also make available OpenTrace, an open-source reference implementation of BlueTrace, on GitHub.
This April 2020 whitepaper authored by Bay et al. of Singapore's Government Technology Agency presents BlueTrace, a privacy-preserving protocol that underpins Singapore's nationally deployed Bluetooth-based contact tracing system TraceTogether. The white paper describes the purpose of BlueTrace as addressing the key limitation of manual contact tracing: "an infected person can only report contacts they are acquainted with and remember having met." They describe the protocol's design considerations and implementation challenges, as well as how it meets interoperability requirements of health authorities considering its use. Finally they address potential security concerns surrounding the tech. They conclude that while not a stand-alone solution to solving contract tracing issues during a pandemic, Bluetooth-based automated tracing instances can still "ultimately coexist and support the pandemic response plans and processes of the public health authorities guiding us through" a pandemic. The authors also make available OpenTrace, an open-source reference implementation of BlueTrace, on GitHub.
 In this 2019 paper published in AIMS Public Health, Bhattacharya et al. argue for the benefits of using blockchain technology in public health surveillance. Noting the transparency and trust aspects of the technology, as well as the benefits of " data security and privacy, cost-effectiveness, and verifiability of data," the authors discuss four primary ways blockchain can be applied to healthcare activities, including community disease surveillance. "Blockchain could help independent organizations to manage data more efficiently during pandemics," they note, particularly in unison with machine learning and AI techniques. They conclude that blockchain-based disease surveillance "can be more effective and rapid than traditional surveillance in terms of coverage, durability, consensus, selective privacy, uniqueness, and timing."
In this 2019 paper published in AIMS Public Health, Bhattacharya et al. argue for the benefits of using blockchain technology in public health surveillance. Noting the transparency and trust aspects of the technology, as well as the benefits of " data security and privacy, cost-effectiveness, and verifiability of data," the authors discuss four primary ways blockchain can be applied to healthcare activities, including community disease surveillance. "Blockchain could help independent organizations to manage data more efficiently during pandemics," they note, particularly in unison with machine learning and AI techniques. They conclude that blockchain-based disease surveillance "can be more effective and rapid than traditional surveillance in terms of coverage, durability, consensus, selective privacy, uniqueness, and timing."
 Cybersecurity remains a vital consideration for entities within the healthcare setting, with academic researchers publishing on the topic frequently. But since computer security in healthcare first started getting researched in the mid-1980s, how have topical focuses shifted? What areas, if any, are underserved by current research? Jalali et al. attempt to answer those and other questions in this 2019 paper published in Journal of Medical Internet Research. Using bibliometric analysis as their tool, the authors conducted chronological analysis, domain clustering analysis, and text analysis on 472 English-language articles ranging from 1985 to September 2017. The conclude that "[d]espite the increase in research and attention to cybersecurity, there are persistent shortcomings in the research on cybersecurity," including implications that emerging cyber threats, physical security methodologies, and several human elements of cybersecurity have not been sufficiently captured in research. They worry that "a school of thought that knowledge, especially specific strategies and tactics, should not be shared openly," further suppresses research growth and utility.
Cybersecurity remains a vital consideration for entities within the healthcare setting, with academic researchers publishing on the topic frequently. But since computer security in healthcare first started getting researched in the mid-1980s, how have topical focuses shifted? What areas, if any, are underserved by current research? Jalali et al. attempt to answer those and other questions in this 2019 paper published in Journal of Medical Internet Research. Using bibliometric analysis as their tool, the authors conducted chronological analysis, domain clustering analysis, and text analysis on 472 English-language articles ranging from 1985 to September 2017. The conclude that "[d]espite the increase in research and attention to cybersecurity, there are persistent shortcomings in the research on cybersecurity," including implications that emerging cyber threats, physical security methodologies, and several human elements of cybersecurity have not been sufficiently captured in research. They worry that "a school of thought that knowledge, especially specific strategies and tactics, should not be shared openly," further suppresses research growth and utility.
 This week we turn back the clock a couple of years to 2018, when Fairchild et al.published in the journal Frontiers in Public Health their analysis of the challenges of epidemiological data reporting. With the march of COVID-19 today, their analysis and advice holds even more relevant today. In their paper, Fairchild et al. first introduced the state of epidemiological data reporting, particularly on the internet. They then discussed the three challenges that affect such reporting: user interface, data format, and data reporting issues. They concluded with nine clear best practices that should be followed when managing epidemiological data for release to the public. They imagined a scenario of a standardized platform adopted worldwide, "where global data could be easily collected without the challenges we currently face. This would in turn streamline epidemiological and public health analysis, modeling, and informatics, resulting in better public health decision-making capabilities."
This week we turn back the clock a couple of years to 2018, when Fairchild et al.published in the journal Frontiers in Public Health their analysis of the challenges of epidemiological data reporting. With the march of COVID-19 today, their analysis and advice holds even more relevant today. In their paper, Fairchild et al. first introduced the state of epidemiological data reporting, particularly on the internet. They then discussed the three challenges that affect such reporting: user interface, data format, and data reporting issues. They concluded with nine clear best practices that should be followed when managing epidemiological data for release to the public. They imagined a scenario of a standardized platform adopted worldwide, "where global data could be easily collected without the challenges we currently face. This would in turn streamline epidemiological and public health analysis, modeling, and informatics, resulting in better public health decision-making capabilities."
 In this brief editorial in the journal Diagnostics, Mashamba-Thompson et al. state their case for a potentially ideal solution to COVID-19 testing in resource-poor communities: using mobile health (mHealth) solutions in conjunction with a blockchain and AI-driven data acquisition and transfer system. They add that the "AI component of this technology enables powerful data collection (patient information, geographic location of the patient, and test results), security, analysis, and curation of disparate and clinical data sets from federated blockchain platforms to derive triangulated data at very high degrees of confidence and speed. " Unfortunately, what isn't clear in their argument is how the self-testing step would actually work, let alone how the system would realistically be implemented in resource-poor settings. One could argue, however, despite the relatively few details, the sharing of ideas on how to address COVID-19 testing in various environments still has value.
In this brief editorial in the journal Diagnostics, Mashamba-Thompson et al. state their case for a potentially ideal solution to COVID-19 testing in resource-poor communities: using mobile health (mHealth) solutions in conjunction with a blockchain and AI-driven data acquisition and transfer system. They add that the "AI component of this technology enables powerful data collection (patient information, geographic location of the patient, and test results), security, analysis, and curation of disparate and clinical data sets from federated blockchain platforms to derive triangulated data at very high degrees of confidence and speed. " Unfortunately, what isn't clear in their argument is how the self-testing step would actually work, let alone how the system would realistically be implemented in resource-poor settings. One could argue, however, despite the relatively few details, the sharing of ideas on how to address COVID-19 testing in various environments still has value.
 In this 2019 paper published in Environmental Health, Udesky et al. share their organization's guidance towards addressing quality in environmental exposure testing and data. In particular, the authors note the importance of reporting quality control data along with chemical measurements in published research, stating that its lacking leaves "readers uncertain about the level of confidence in the reported data." Their objective was to provide full guidance on the implementation, interpretation, and reporting of QC data. After explaining their approach to study design, study implementation, and data interpretation, the authors conclude that their guidance, visualizations, and supplementary content provide "a useful set of tools for getting the best information from valuable environmental exposure datasets, and enabling valid comparison and synthesis of exposure data across studies."
In this 2019 paper published in Environmental Health, Udesky et al. share their organization's guidance towards addressing quality in environmental exposure testing and data. In particular, the authors note the importance of reporting quality control data along with chemical measurements in published research, stating that its lacking leaves "readers uncertain about the level of confidence in the reported data." Their objective was to provide full guidance on the implementation, interpretation, and reporting of QC data. After explaining their approach to study design, study implementation, and data interpretation, the authors conclude that their guidance, visualizations, and supplementary content provide "a useful set of tools for getting the best information from valuable environmental exposure datasets, and enabling valid comparison and synthesis of exposure data across studies."
 In this March 2020 journal article published in the journal Micromachines, Nguyen, Bang, and Wolff give their professional take on point-of-care diagnostics for combating the COVID-19 epidemic. In particular, the authors discuss the loop-mediated isothermal amplification (LAMP) nucleic acid amplification technique and its potential to make COVID-19 testing faster, more portable, more flexible, more stable, and more sensitive. After discussing the then-recent events (information is changing rapidly), the authors compare real-time reverse transcription polymerase chain reaction (rRT-PCR) with LAMP methods and discuss other important factors affecting society because of the pandemic.
In this March 2020 journal article published in the journal Micromachines, Nguyen, Bang, and Wolff give their professional take on point-of-care diagnostics for combating the COVID-19 epidemic. In particular, the authors discuss the loop-mediated isothermal amplification (LAMP) nucleic acid amplification technique and its potential to make COVID-19 testing faster, more portable, more flexible, more stable, and more sensitive. After discussing the then-recent events (information is changing rapidly), the authors compare real-time reverse transcription polymerase chain reaction (rRT-PCR) with LAMP methods and discuss other important factors affecting society because of the pandemic.
 In this brief editorial article published in the journal Diagnostics, Tiawanese researchers Yang et al. provide their insights into a more convenient at-home point-of-care (POC) testing method for the COVID-19 illness. Noting the urgency of the associated pandemic and the various costs associated with traditional testing methodologies such as lateral flow immunoassays and molecular-based assays, the authors suggest the blending of several tools: a paper-based result and common mobile devices. A patient at home could take a nasal swab and use a POC device to return a colorimetric result, which could then be captured by mobile phone and rapidly sent to a clinician for analysis. They conclude that such a method could "provide new insights into designing POC COVID-19 diagnostics and ultimately improve the health care system to combat this and similar diseases."
In this brief editorial article published in the journal Diagnostics, Tiawanese researchers Yang et al. provide their insights into a more convenient at-home point-of-care (POC) testing method for the COVID-19 illness. Noting the urgency of the associated pandemic and the various costs associated with traditional testing methodologies such as lateral flow immunoassays and molecular-based assays, the authors suggest the blending of several tools: a paper-based result and common mobile devices. A patient at home could take a nasal swab and use a POC device to return a colorimetric result, which could then be captured by mobile phone and rapidly sent to a clinician for analysis. They conclude that such a method could "provide new insights into designing POC COVID-19 diagnostics and ultimately improve the health care system to combat this and similar diseases."
 This is the third of a series of interim guidance documents, this one originating from the U.S. Food and Drug Administration (FDA). Issued on March 16, this guidance document provides insight into FDA policy for the development, validation, and use of diagnostic tests for Coronavirus Disease-2019 (COVID-19), particularly those tests issued an Emergency Use Authorization (EUA). This guidance applies to not only manufacturers developing and distributing test kits but also CLIA laboratories cleared for high-complexity testing that develop and validate their own tests. The FDA provides background on the topic and then discusses the policy in detail, before closing with its validation study recommendations.
This is the third of a series of interim guidance documents, this one originating from the U.S. Food and Drug Administration (FDA). Issued on March 16, this guidance document provides insight into FDA policy for the development, validation, and use of diagnostic tests for Coronavirus Disease-2019 (COVID-19), particularly those tests issued an Emergency Use Authorization (EUA). This guidance applies to not only manufacturers developing and distributing test kits but also CLIA laboratories cleared for high-complexity testing that develop and validate their own tests. The FDA provides background on the topic and then discusses the policy in detail, before closing with its validation study recommendations.
 Again, not a journal article, but here rather we have another interim guidance document by the World Health Organization (WHO), this time in regards to laboratory biosafety related to COVID-19 testing. Effective March 19, 2020, this guidance document, complete with references, addresses laboratory biosafety principles to consider when handling and testing specimens believed to contain the COVID-19 virus. The WHO provides a bit of background, provides an overview of the key points, and then discusses those points in further detail. They also include two annex items: the core requirements of good microbiological practice and procedure (GMPP) and a risk assessment template for laboratories to borrow from.
Again, not a journal article, but here rather we have another interim guidance document by the World Health Organization (WHO), this time in regards to laboratory biosafety related to COVID-19 testing. Effective March 19, 2020, this guidance document, complete with references, addresses laboratory biosafety principles to consider when handling and testing specimens believed to contain the COVID-19 virus. The WHO provides a bit of background, provides an overview of the key points, and then discusses those points in further detail. They also include two annex items: the core requirements of good microbiological practice and procedure (GMPP) and a risk assessment template for laboratories to borrow from.
 Breaking from the norm, rather than a journal article, this document represents official interim guidance on laboratory testing for COVID-19 from the World Health Organization (WHO), effective March 19, 2020. This guidance document, complete with references, addresses laboratory testing guidance principles for testing patients who meet the case definition for being infected with the COVID-19 virus. The WHO provides a bit of background, then addresses specimen collection and shipment, laboratory testing procedures, and reporting. They also include where further research must tread, as well as an ISO 15189:2012-compliant COVID-19 laboratory test request form. WHO closes by encouraging "the sharing of data to better understand and thus manage the COVID-19 outbreak, and to develop countermeasures."
Breaking from the norm, rather than a journal article, this document represents official interim guidance on laboratory testing for COVID-19 from the World Health Organization (WHO), effective March 19, 2020. This guidance document, complete with references, addresses laboratory testing guidance principles for testing patients who meet the case definition for being infected with the COVID-19 virus. The WHO provides a bit of background, then addresses specimen collection and shipment, laboratory testing procedures, and reporting. They also include where further research must tread, as well as an ISO 15189:2012-compliant COVID-19 laboratory test request form. WHO closes by encouraging "the sharing of data to better understand and thus manage the COVID-19 outbreak, and to develop countermeasures."
 In this 2019 journal article published in the African Journal of Laboratory Medicine, Mtonga et al. describe the efforts of developing a laboratory information system (LIS) for resource-poor regions, as well the benefits of other informatics interventions in those settings. Using open-source C4G BLIS as their base, the researchers then turned to "user stories" as a recommended method for determining the system requirements the end users required, then updating with those requirements as well as critical functionality that would suitable for resource-poor hospitals and labs. The authors then deployed the resulting LIS in Kamuzu Central Hospital in Lilongwe, Malawi. They conclude their experience "highlights the potential of using informatics interventions to address systemic problems in the laboratory testing process in low-resource settings," though the implementation of those interventions "may require innovation of new hardware to address various contextual issues."
In this 2019 journal article published in the African Journal of Laboratory Medicine, Mtonga et al. describe the efforts of developing a laboratory information system (LIS) for resource-poor regions, as well the benefits of other informatics interventions in those settings. Using open-source C4G BLIS as their base, the researchers then turned to "user stories" as a recommended method for determining the system requirements the end users required, then updating with those requirements as well as critical functionality that would suitable for resource-poor hospitals and labs. The authors then deployed the resulting LIS in Kamuzu Central Hospital in Lilongwe, Malawi. They conclude their experience "highlights the potential of using informatics interventions to address systemic problems in the laboratory testing process in low-resource settings," though the implementation of those interventions "may require innovation of new hardware to address various contextual issues."
 In this 2020 paper published in the journal Toxins, Narváez et al. of Spain and Italy present the results of their attempt to use a specific type of chromatography and spectrometry in order to analyze the amount of mycotoxins and pesticides in nutraceuticals made from Cannabis sativa and other botanicals. The authors developed specific procedures associated with a "methodology using ultra-high-performance liquid chromatography coupled with quadrupole-Orbitrap high-resolution mass spectrometry (UHPLC-Q-Orbitrap HRMS)." After testing their methodology on 10 cannabidiol (CBD) supplements, they found numerous mycotoxins and dozens of pesticides. They conclude that their "results highlight the necessity of monitoring contaminants in food supplements in order to ensure safe consumption" while also demonstrating the effectiveness of their methodology in doing so.
In this 2020 paper published in the journal Toxins, Narváez et al. of Spain and Italy present the results of their attempt to use a specific type of chromatography and spectrometry in order to analyze the amount of mycotoxins and pesticides in nutraceuticals made from Cannabis sativa and other botanicals. The authors developed specific procedures associated with a "methodology using ultra-high-performance liquid chromatography coupled with quadrupole-Orbitrap high-resolution mass spectrometry (UHPLC-Q-Orbitrap HRMS)." After testing their methodology on 10 cannabidiol (CBD) supplements, they found numerous mycotoxins and dozens of pesticides. They conclude that their "results highlight the necessity of monitoring contaminants in food supplements in order to ensure safe consumption" while also demonstrating the effectiveness of their methodology in doing so.
 Cybersecurity is an increasingly relevant and critical topic for most industries, including the healthcare industry. In hospitals and other clinical settings, patient monitoring systems, medical imaging systems, and laboratory instruments are increasingly networked to get the most out of data management and operational efficiency. However, these devices are at risk of cyber attack, with potentially significant consequences. Johansson et al. acknowledge this problem and attempt to address the perceptions of information and medical technology experts on the topic of securing in-house medical devices through the practice of network segmentation. The authors present their discussion results with 18 stakeholders from the Swedish Region Skåne, and they conclude that the stakeholders "were positively receptive to the increase in cybersecurity provided by network segmentation but concerned about the increase in the administration that it will entail for medical devices." They note that these and other opinions stated in the research are vital for health networks to take into account when considering improving their cybersecurity.
Cybersecurity is an increasingly relevant and critical topic for most industries, including the healthcare industry. In hospitals and other clinical settings, patient monitoring systems, medical imaging systems, and laboratory instruments are increasingly networked to get the most out of data management and operational efficiency. However, these devices are at risk of cyber attack, with potentially significant consequences. Johansson et al. acknowledge this problem and attempt to address the perceptions of information and medical technology experts on the topic of securing in-house medical devices through the practice of network segmentation. The authors present their discussion results with 18 stakeholders from the Swedish Region Skåne, and they conclude that the stakeholders "were positively receptive to the increase in cybersecurity provided by network segmentation but concerned about the increase in the administration that it will entail for medical devices." They note that these and other opinions stated in the research are vital for health networks to take into account when considering improving their cybersecurity.
 Ontologies are important to biological and biomedical researchers working with multiple data sources because, when implemented well, ontologies ensure heterogeneous, multimodal data across multiple domains and they aid with more effective data mining and discoveries. Ontologies also assist in improving data quality. In this 2019 paper, Jackson et al. highlight the importance of ontologies with their ROBOT open-source library, which aids with the automation of ontology development. They describe the various means which ROBOT "provides ontology processing commands for a variety of tasks, including commands for converting formats, running a reasoner, creating import modules, running reports, and various other tasks." They conclude that such automation allows developers to easily "configure, combine, and execute individual tasks in comprehensive, automated workflows." The authors also demonstrate how ROBOT can help catch logical errors and provide quality control mechanisms to ontology creation and updating.
Ontologies are important to biological and biomedical researchers working with multiple data sources because, when implemented well, ontologies ensure heterogeneous, multimodal data across multiple domains and they aid with more effective data mining and discoveries. Ontologies also assist in improving data quality. In this 2019 paper, Jackson et al. highlight the importance of ontologies with their ROBOT open-source library, which aids with the automation of ontology development. They describe the various means which ROBOT "provides ontology processing commands for a variety of tasks, including commands for converting formats, running a reasoner, creating import modules, running reports, and various other tasks." They conclude that such automation allows developers to easily "configure, combine, and execute individual tasks in comprehensive, automated workflows." The authors also demonstrate how ROBOT can help catch logical errors and provide quality control mechanisms to ontology creation and updating.
 In this brief article by Schmieder et al., the authors provide an example of applying laboratory informatics to research activities for drug discovery and personalized medicine. In particular, the authors look at the in vitro nephrotoxicity assay and the use of transepithelial electrical resistance (TEER) to evaluate the barrier function of cellular layers. Citing issues such as the significant multi-step processes involved with this type of testing, the authors attempt to demonstrate how a laboratory information management system (LIMS), laboratory execution system (LES), and automation standards such as SiLA can be used together to decrease the workflow complexity of such cell-based assays.
In this brief article by Schmieder et al., the authors provide an example of applying laboratory informatics to research activities for drug discovery and personalized medicine. In particular, the authors look at the in vitro nephrotoxicity assay and the use of transepithelial electrical resistance (TEER) to evaluate the barrier function of cellular layers. Citing issues such as the significant multi-step processes involved with this type of testing, the authors attempt to demonstrate how a laboratory information management system (LIMS), laboratory execution system (LES), and automation standards such as SiLA can be used together to decrease the workflow complexity of such cell-based assays.
















