Posted on December 21, 2021
By LabLynx
Journal articles
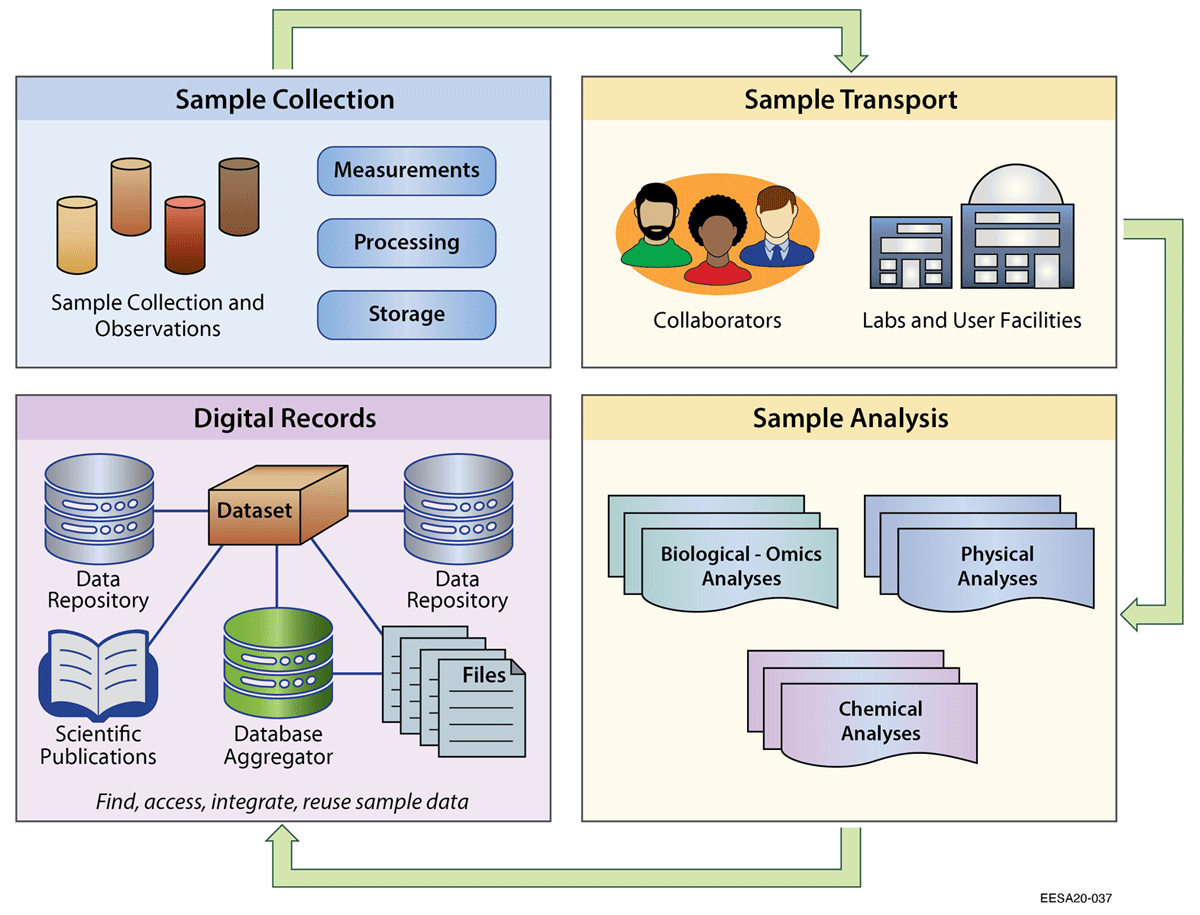
In this 2021 journal article published in
Data Science Journal, Damerow
et al. emphasize that while sample-based research is critical to a wide range of ecosystem sciences, the increasingly multidisciplinary approach to those sciences requires a better, more coordinated practice of sample identification and data management. "While there are widely adopted conventions within certain domains to describe sample data," they say, "these have gaps when applied in a multidisciplinary context." With this paper, the authors present a more practical approach to sample identification and management that takes into account the multidisciplinary requirements of ecosystems research. After a brief review of literature and existing sample identification methods, guidance, and standards, they describe their pilot program for standardizing sample metadata and propose several benefits to the program. They conclude that "user-friendly guidance and sample metadata templates are an essential step in promoting standard practices that make data publishing, integration, and reuse easier," though proper training, legacy data management tools, and information management systems are also important components towards those goals.
Posted on December 15, 2021
By LabLynx
Journal articles
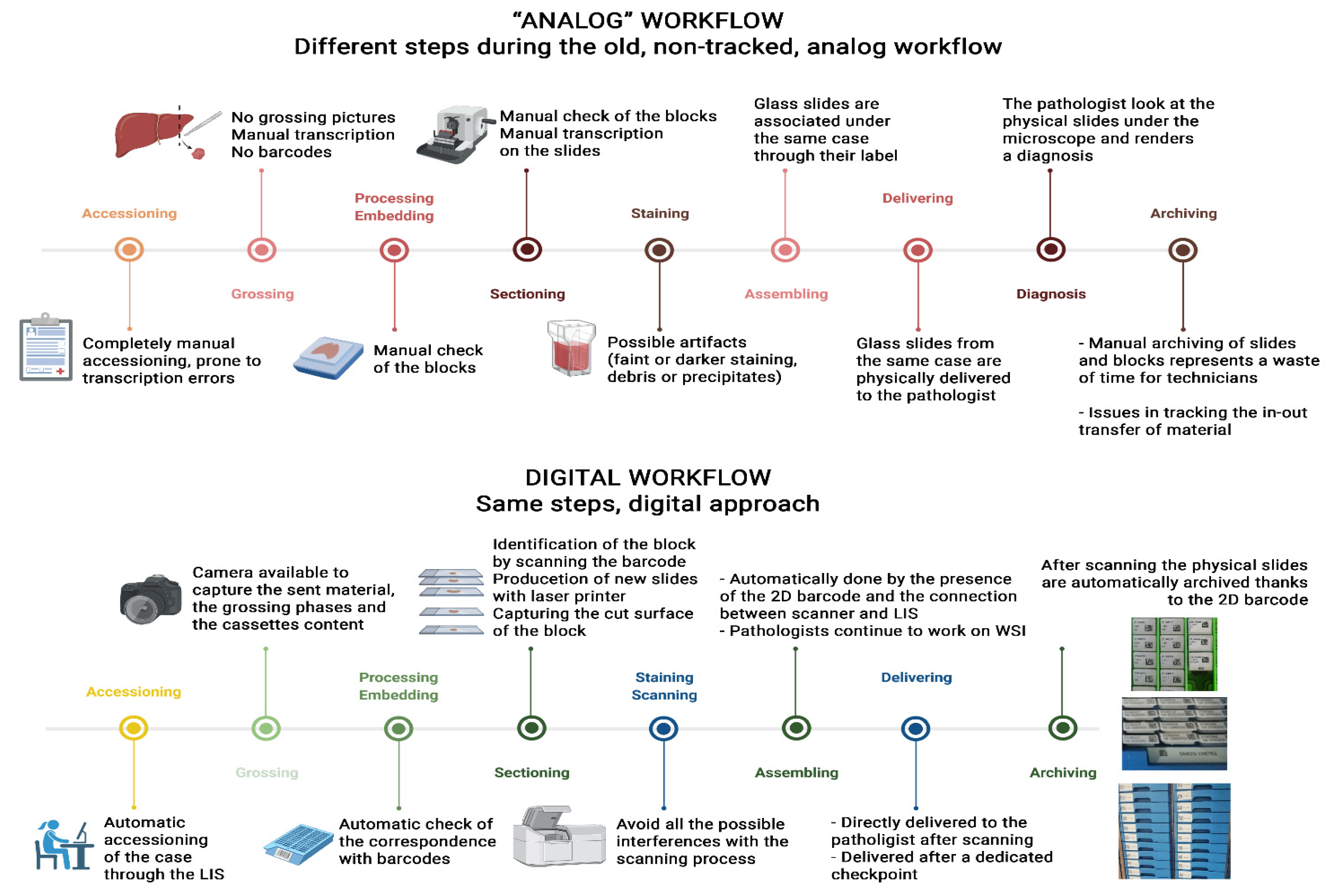
The implementation of digital pathology workflows has seen an uptick in interest in recent years, though as Fraggetta
et al. point out in this 2021 journal article, only a minority of pathology laboratories have fully embraced these workflows. Wanting to increase the adoption rate of digital pathology, the authors present their experiences with digital pathology and focus on four critical considerations to improve adoption rates. After presenting a brief introduction to digital pathology, the authors discuss various aspects of involvement, optimization, and automation required to get digital pathology projects initially started. They then commit a significant portion of their paper to discussing the quality control program that must be implemented as part of a digital pathology workflow. They also address whole slide imaging and its validation, as well as retention policies, results evaluation, and the necessary maintenance of workflows after implementation. They conclude with 10 basic principles (classified as recommendations or suggestions) to address when transitioning from "a classic, 'analog' to a completely digital workflow," adding that " the present document represents a practical, handy reference for the correct implementation of a digital workflow in Europe."
Posted on December 6, 2021
By LabLynx
Journal articles
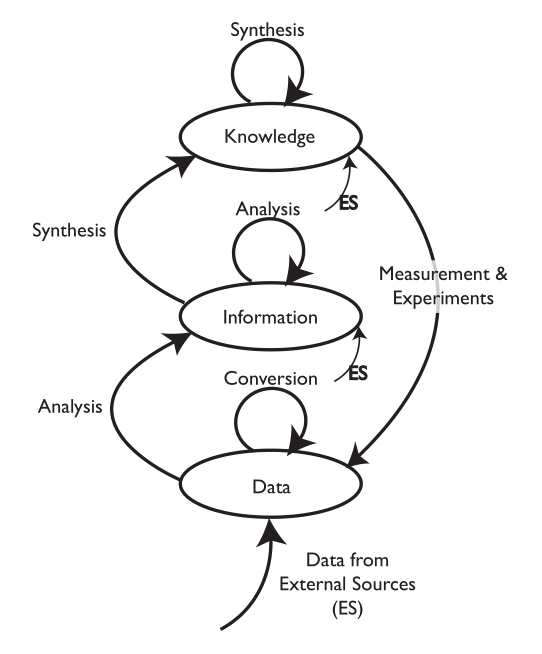
Veteran laboratory automation/computing professional Joe Liscouski is at it again, this time releasing a perspective piece that takes elements from more than 15 years of writing and presentation, painting a nuanced approach to planning for the use of computer systems in the laboratory. In particular, this November 2021 work continues to expand on the importance of laboratory systems engineering in the laboratory of the future. After providing a full introduction, Liscouski examines both the past and present of laboratory computing and how the automation aspects of that computing affects laboratory personnel. He then goes on to espouse the benefits of a more industry-wide approach to addressing the technological and educational needs of laboratories of all types, particularly in regard to how standardization plays an important role. He then addresses laboratory work itself, how automation can move that work forward, and how to effectively apply that automation to the laboratory. Finally, Liscouski closes by emphasizing the importance of a "center for laboratory systems engineering" to help centralize the efforts mentioned in the guide. A sizeable appendix is included, providing more historical perspective to the work and its conclusions.
Posted on November 29, 2021
By LabLynx
Journal articles
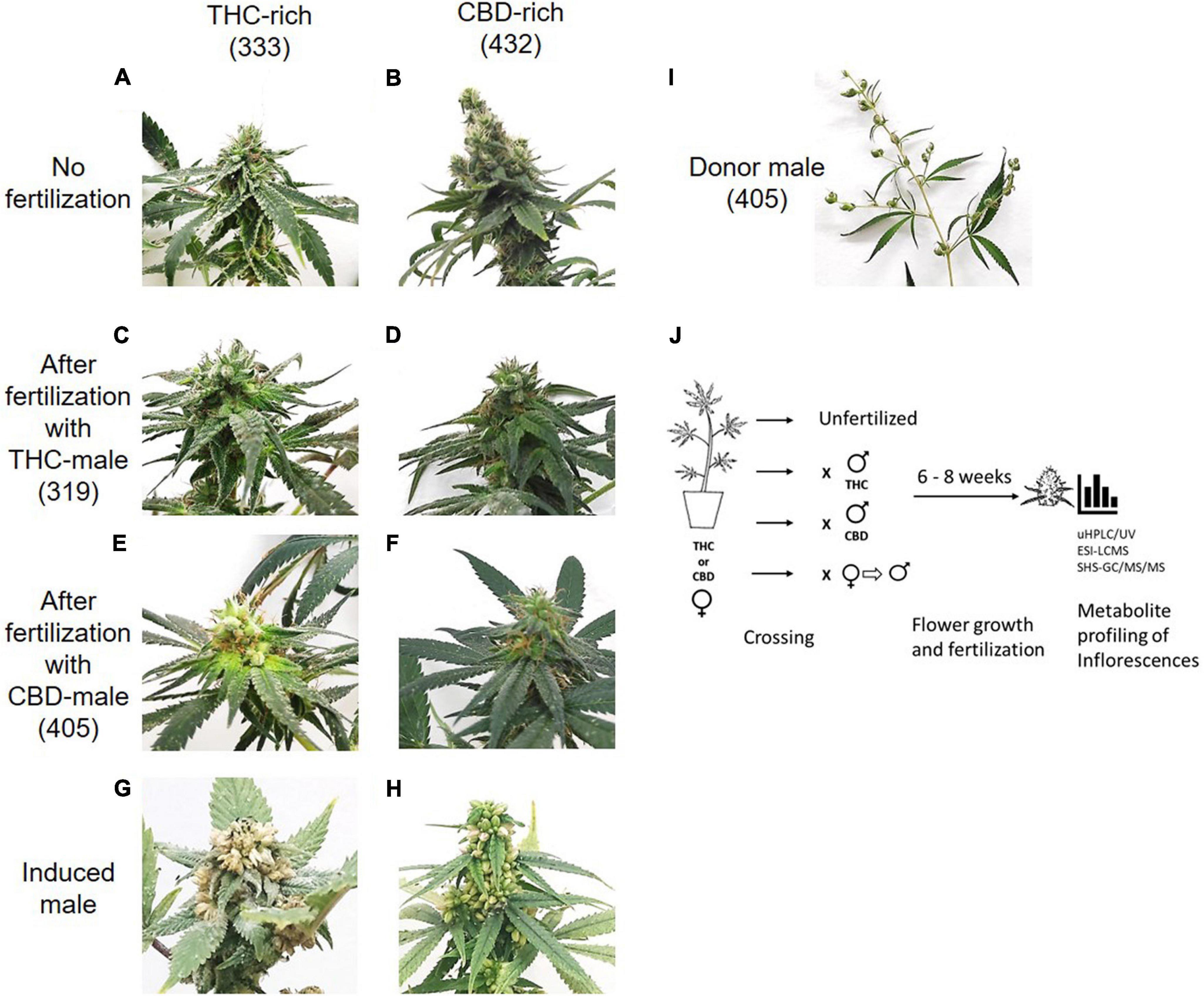
In this 2021 paper published in
Frontiers in Plant Science, Feder
et al. present the analytical results of
Cannabis inflorescences that have been pollinated and fertilized, in an attempt to show that fertilization can affect phytocannabinoid accumulation, among other goals. Noting that growers have recently shifted to using seeds, which can despite feminization reveal to be male seeds five to ten percent of the time, the authors note that this type of research—rarely conducted—is particularly critical to answering questions about how pollination effects phytocannabinoid and terpenoid expression. After providing background information and reviewing materials and methods for their analyses, the authors discuss the results, noting in particular that "phytocannabinoid quantity predominately decreases after fertilization," terpenoid quantity can vary based upon the type of female plant, and that "individual terpenoid concentrations are differentially affected by fertilization." After further discussion, they conclude not only the three prior-mentioned findings, but they also by extension suggest that their findings indicate the "functional roles" of phytocannabinoids and terpenoids in
Cannabis's plant life cycle.
Posted on November 23, 2021
By LabLynx
Journal articles
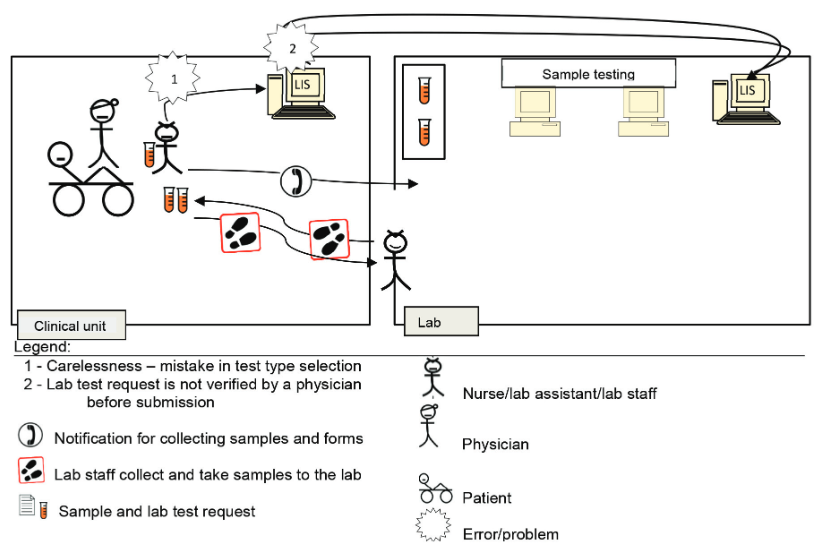
In this 2021 journal article published in
Journal of Medical Biochemistry, Arifin and Yusof of Universiti Kebangsaan Malaysia examine the error factors that come with using a laboratory information system (LIS) and propose the "total testing process for laboratory information systems" (TTP-LIS). This process leans on a variety of existing frameworks and lean quality improvement methods to meet the authors' needs and is applied to two large hospitals in Malaysia. After examining human, technology, and organizational factors, the authors discuss their findings, noting that their "findings showed the practicality of the TTP-LIS framework as an evaluation tool in identifying errors and their causal factors. The use of lean tools—namely, VSM, A3, and 5 Why—enabled us to analyze and visualize the root cause of problems in an objective and structured manner. " Those root causes were able to be categorized in three ways: "as a latent failure in system development, as poor error management, and as unsatisfactory lab testing processes and LIS use."
Posted on November 16, 2021
By LabLynx
Journal articles
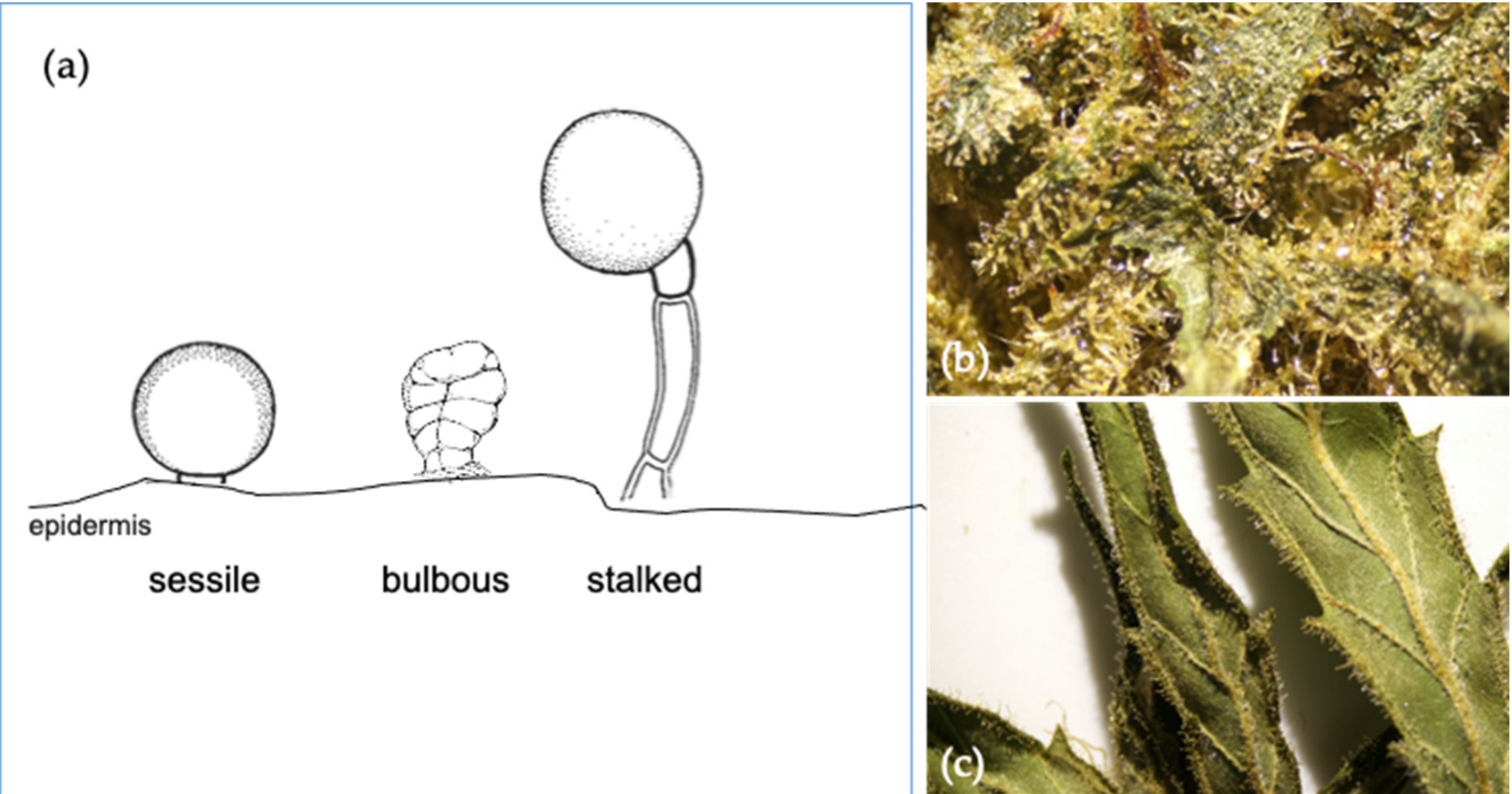
When it comes to analyzing the
Cannabis plant and its constituents, terpenes are one such component that hasn't been well studied until recent years. But as Sommano
et al. note in their 2020 paper published in
Molecules, "a growing number of industries have shown interest in adding either cannabis terpenes or botanically-derived terpenes to their CBD oils and edibles." This has resulted in demand for further study of terpenes and how they interact with other constituents. The authors present a broad look at terpenes in their paper. examining the taxonomy and localization of terpenes from
Cannabis plants, their biosynthesis, and their prevalence in certain chemovars. They close by briefly discussing separation of terpenes, and concluding that "terpene profiles not only embody the characteristics of cannabis genotypes, but their entourage effect with cannabinoids could enhance their medicinal functionality."
Posted on November 9, 2021
By LabLynx
Journal articles
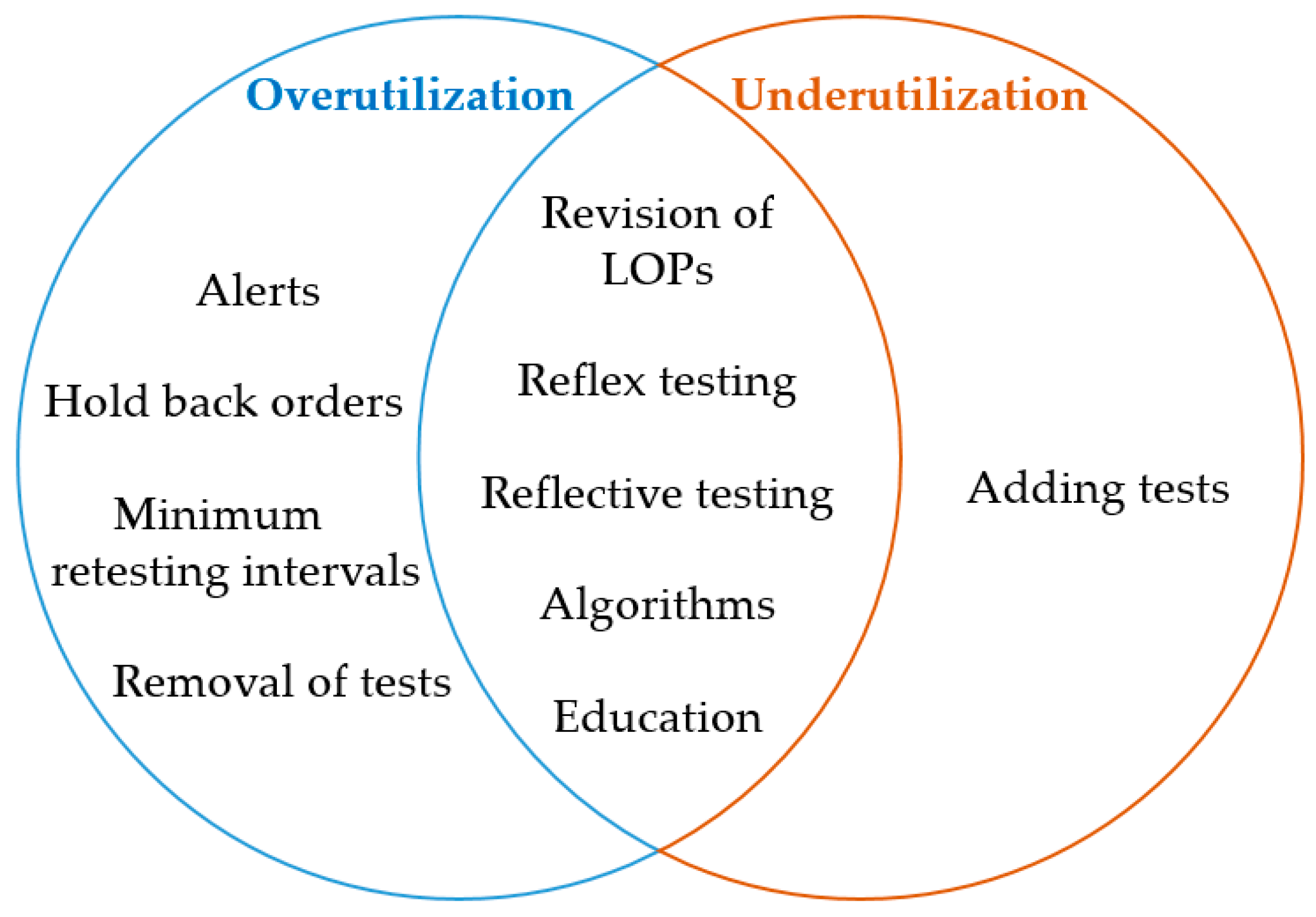
In this 2021 article published in the journal
Diagnostics, Mrazek
et al. of the Paracelsus Medical University provide a review of the available demand management (DM) strategies available to laboratorians and clinicians for avoiding inappropriate utilization of specific laboratory diagnostics. The authors provide 10 potential strategies toward that goal, including the likes of more practical order entry alerts, better utilization of the laboratory information system (LIS), outdated test removal, relevant test addition, and artificial intelligence (AI) algorithms, to name a few. However, whatever methods are implemented, the authors note that it's vital for demand management strategies "to be adapted to local settings." Additionally, given that many of the strategies may be unduly time-consuming, they "believe that AI solutions are the next logical step, aiding in the development as well as improvement of DM strategies, as they could help to manage large data sets." However, even AI has its caveats, as "a tool of assistance," they add.
Posted on November 2, 2021
By LabLynx
Journal articles
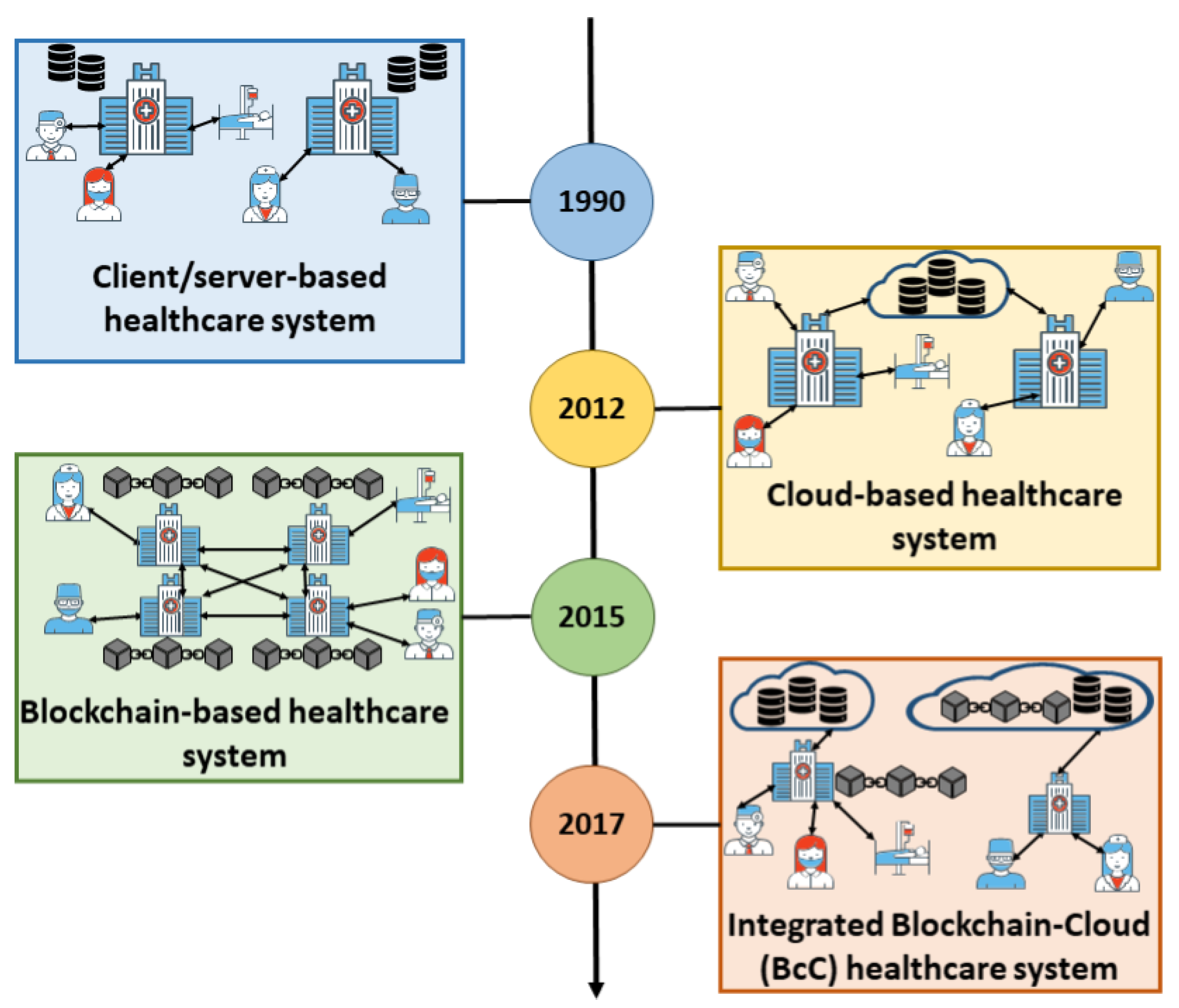
As laboratories continue to swim in an increasing amount of data and attempt to streamline operations, thoughts of turning to cloud-based data management grow. And while cloud-based applications can help, challenges remain with security, privacy, and real-time access. Blockchain-based systems alone can improve on some of those challenges, but blockchain isn't scalable. As such, Ismail
et al. discuss at length in this 2021 paper the benefits of integrating blockchain with cloud-computing systems, as well as the remaining challenges of optimizing such an integration. After presenting existing research and discussing the basics of cloud computing and blockchain, the authors discuss the integration of the two in both encapsulated- and non-encapsulated architectures, as well as how they can be applied to healthcare systems. They close by addressing the remaining challenges of an integrated system, including the energy costs of running such a system. They conclude that while "the integration of cloud and blockchain for healthcare is promising to cope with the shortcomings of these individual technologies ... further research is still required to enhance the existing architecture to make it more scalable and energy-efficient with inter-cloud communication support."
Posted on October 26, 2021
By LabLynx
Journal articles
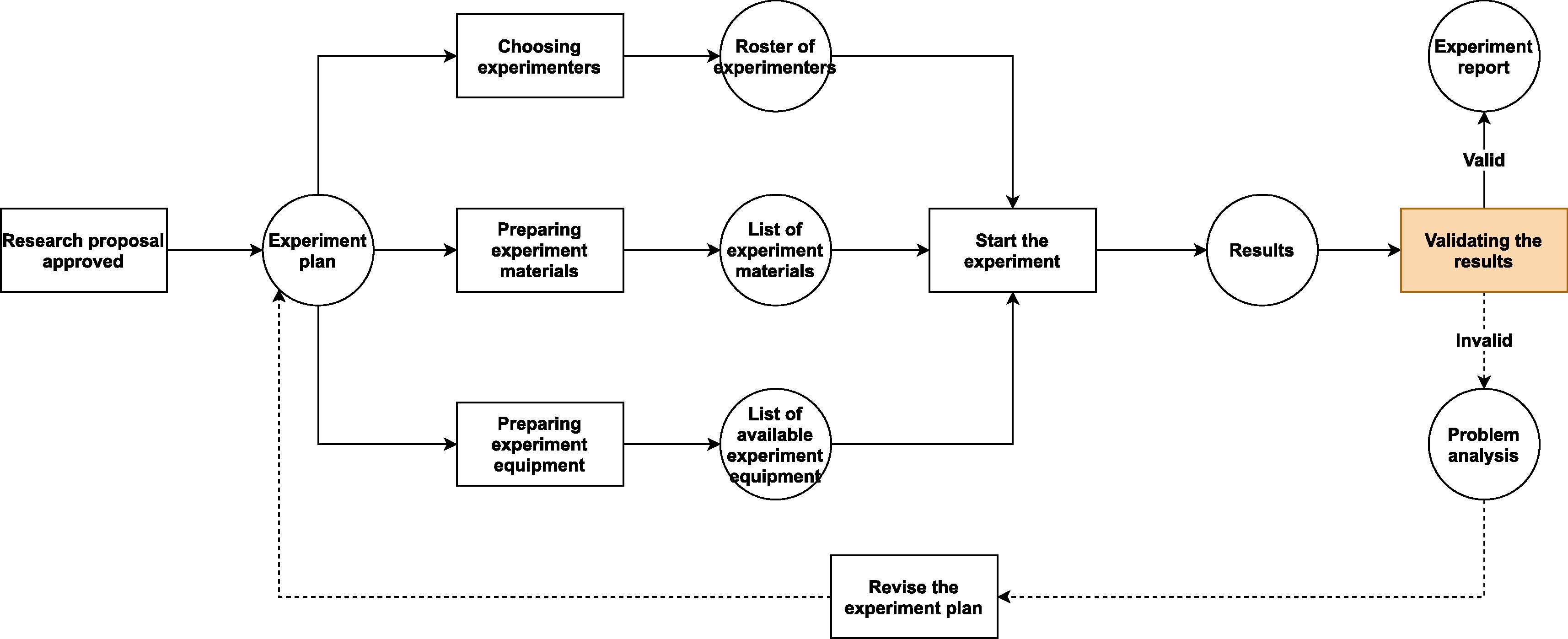
There are numerous laboratory information management systems (LIMS) on the market, but in reality, how many are finely tuned to handle the safety requirements of a biosafety laboratory? At least from the standpoint of Sun
et al. of the Institute of Microbiology Chinese Academy of Sciences, few if any meet those needs. As such, the authors, in this 2021 paper published in the
Journal of Biosafety and Biosecurity, commence with laying out the unique add-on requirements a standard LIMS would require in order to meet the needs of most biosafety lab. Noting in their introduction "a strange situation, one where biosafety information itself is highly digitalized, yet there is no centralized system designed to better organize that electronic information and enable laboratorians to use it appropriately," the authors then describe what is required to remedy that situation. They note that biosafety and efficiency are tied to four information management aspects of the biosafety lab: project management, personnel administration, experimental material management, and equipment management. The authors then discuss how these areas of management would need to be fortified in a modern LIMS, as well as addressing other areas such as balancing flexibility and complexity and addressing data security.
Posted on October 19, 2021
By LabLynx
Journal articles
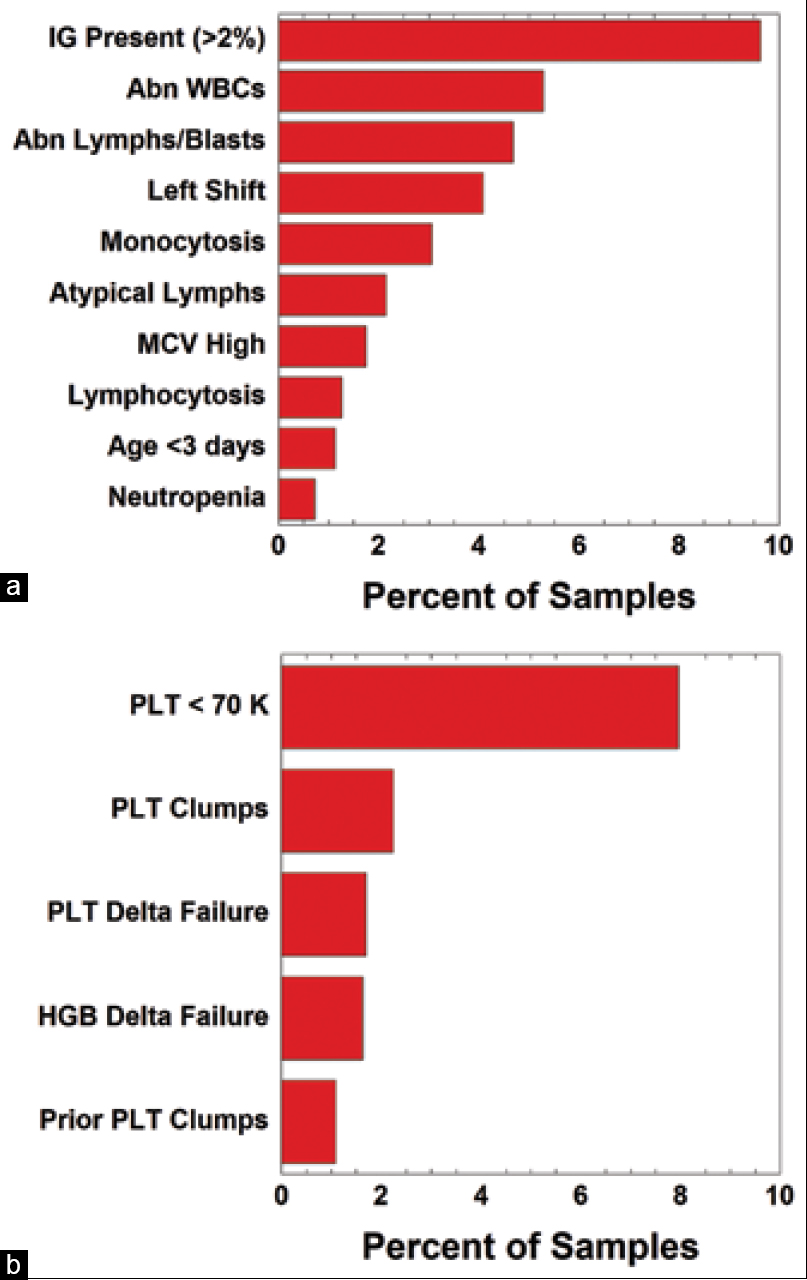
In this 2021 journal article published in the
Journal of Pathology Informatics, Starks
et al. of the University of Iowa Hospitals and Clinics present their case for the value of autoverification data evaluation despite the inherent challenges of extracting and analyzing data related to autoverification rules from informatics system. Noting that most autoverification rules were housed in their middleware solution and (and not the LIS, which would require additional fields currently not present), they examined instrument- and middleware-generated flags generated from "complete blood count (CBC) with white blood cell (WBC) count differential (Diff) and the '
a la carte' ordering of individual CBC components." Their analysis resulted in two significant changes to their autoverification rules related to those tests, resulting in "improved efficiency and lower rerun rates." However, these insights did come at some investment in time and resources, requiring third-party data retrieval methods and "extensive cleanup and formatting" of the data to actually identify flagged specimens. The authors conclude that these downsides highlight "opportunities for improvement in software tools that allow for more rapid and routine evaluation of autoverification parameters."
Posted on October 12, 2021
By LabLynx
Journal articles
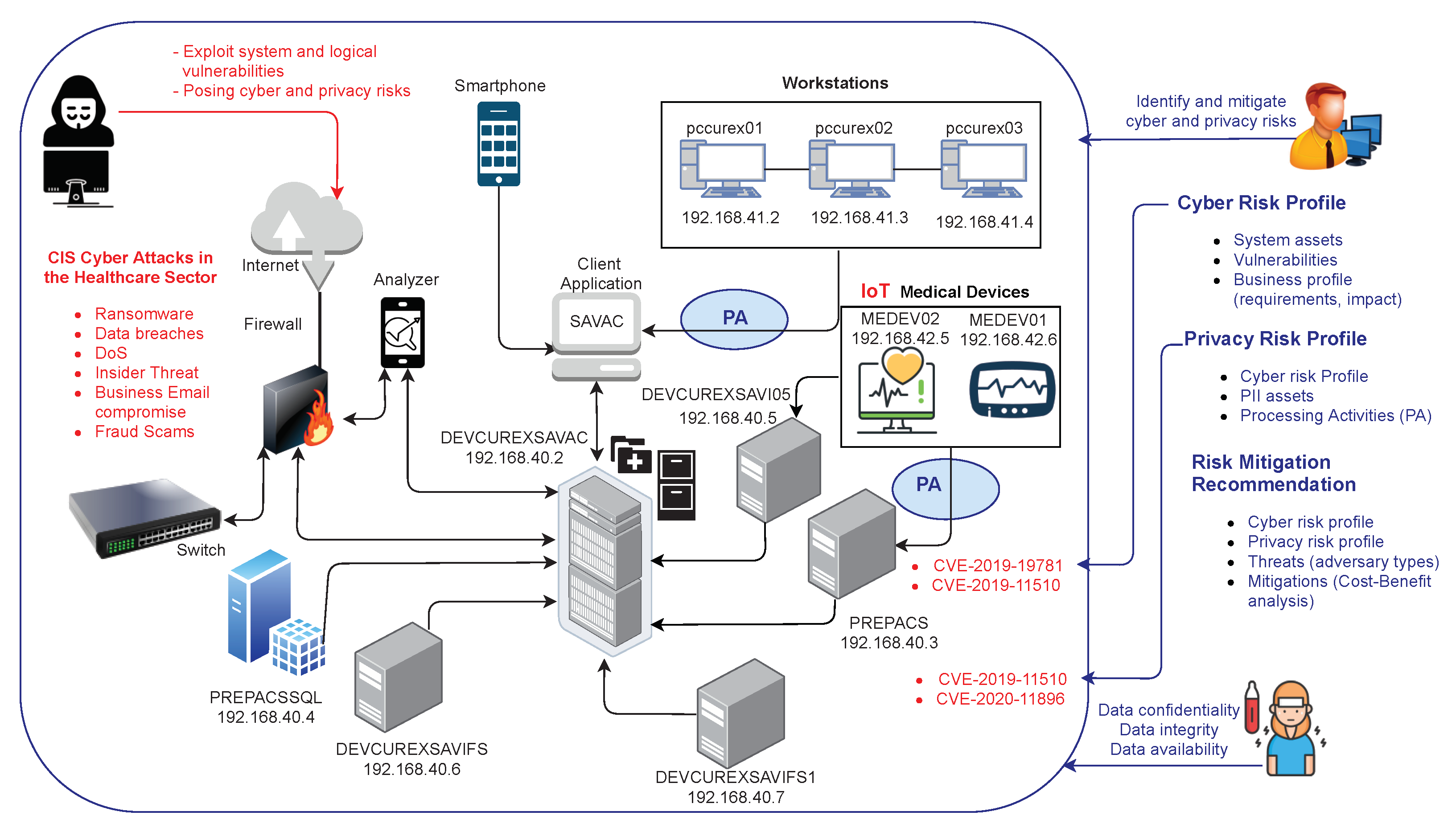
Managing cybersecurity within an organization can be challenging, more so when the in-house knowledge and management drive concerning cybersecurity is lacking. But does it have to be so complicated? What tools exist to help organizations sort through and implement proper safeguards for their cyber infrastructure, and in particular their associated privacy-specific data? In this 2021 paper published in
Sensors, Gonzalez-Granadillo
et al. propose a privacy risk management toolkit called AMBIENT (Automated Cyber and Privacy Risk Management Toolkit) that "not only assesses cyber and privacy risks in a thorough and automated manner, but it also offers decision-support capabilities to recommend optimal safeguards using the well-known repository of the Center for Internet Security (CIS) Controls." After presenting related work, describing AMBIENT, and showing the results of a demo, the author conclude that "security managers can use AMBIENT’s results as a guide in their decision-making process to define appropriate security policies and strategies that keep risk scores within acceptable levels."
Posted on October 5, 2021
By LabLynx
Journal articles
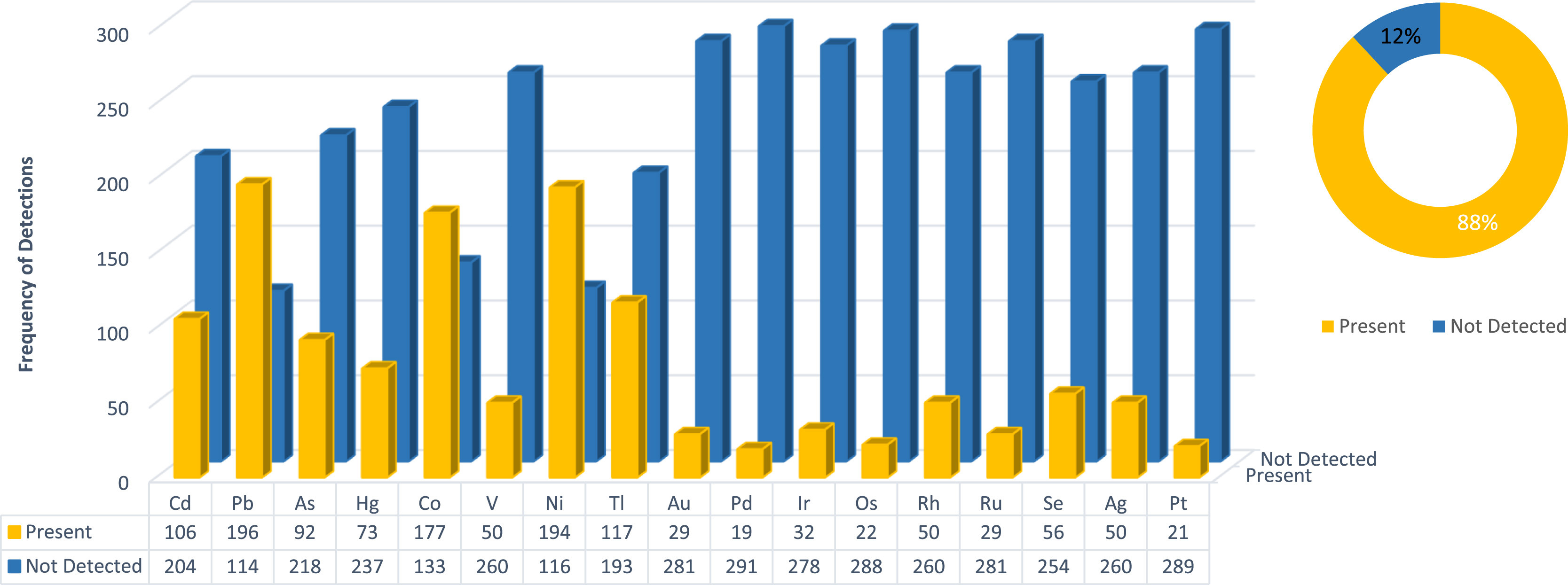
In this 2021 article published in
Forensic Science International Reports, Viviers
et al. present their findings of a wide assessment of heavy metals content in South African cannabis products. Noting a dearth of such research in the South African market, the authors acquired 310 samples (representing seven different sample types) from "cultivators of plants, producers of products, importers, resellers, and pharmaceutical manufacturers" and had them analyzed for their various residues. The authors turned to inductively coupled plasma mass spectrometry (ICP-MS), the
United States Pharmacopeia (
USP) <232>/<233> and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q3D to guide their analyses. After a brief discussion of materials and methods, the authors present their results and conclusions. They found "among all residues analyzed, a Class 1 residue will be present in a third of all samples" (cadmium, lead, arsenic, and mercury), 15% of all samples failed heavy metal analysis "when compared against the
USP <232>/<233> and ICH Q3D oral specification limit," and 44% failed when compared to the specified inhalation limits. The conclude that "when considering the results obtained, it is exceedingly necessary to have an appropriate quality control system in place, especially for heavy metal residues analysis in the current South African market. "
Posted on September 28, 2021
By LabLynx
Journal articles
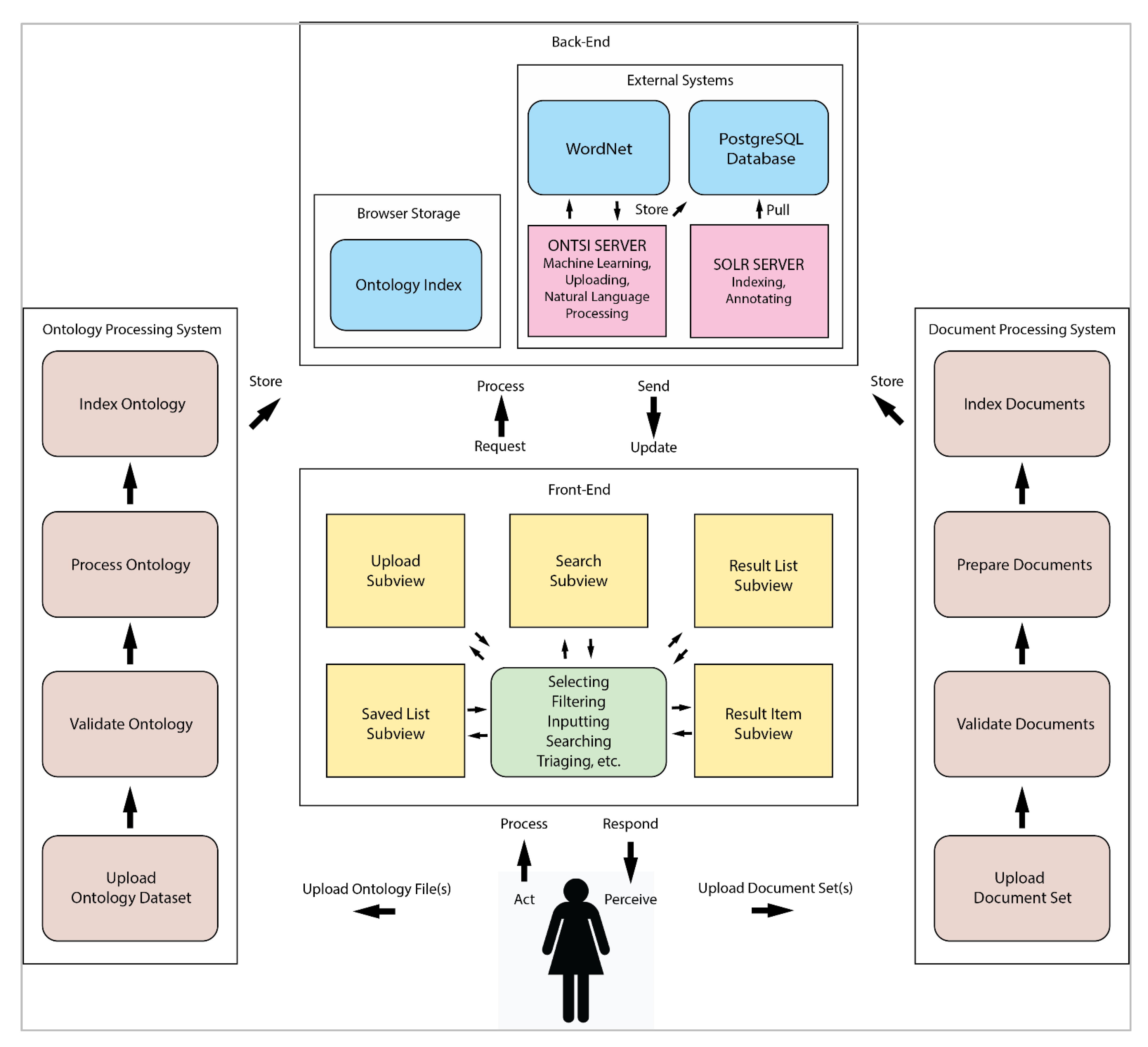
Using computer-based resources to find and retrieve healthcare information is increasingly common, whether it's the healthcare professional or a patient. But "search can be challenging, particularly for health informatics tasks that utilize large and complex document sets," notes Demelo and Sedig in their 2021 paper published in
Information. This led the authors to examine the current state of healthcare informatics and develop an ontology-based search interface called ONTSI. Based on insights from previously published research on health informatics information retrieval, the authors discuss the criteria, design elements, and strategies they used to develop ONTSI. After explaining the results of their work, via a use case, the authors conclude that their tool "ONTSI supplies a generalized interface that supports users’ ability to plug-and-play their provided document sets and an ontology file as a mediating resource within the interface when performing their health informatics search tasks."
Posted on September 22, 2021
By LabLynx
Journal articles
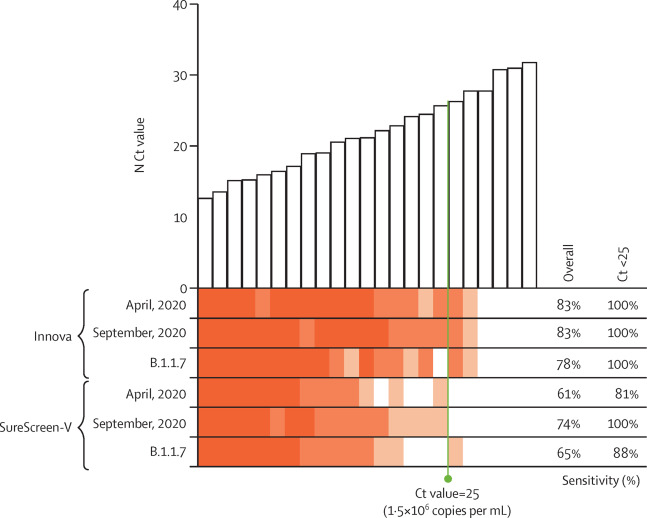
In this 2021 article published in the journal
The Lancet Microbe, Pickering
et al. present their findings concerning the comparison of antigen lateral flow devices (LFDs) for COVID-19 and virus infectivity, noting their study is "the largest to date assessing the correlation between [cycle threshold] values, quantitative culture of infectious virus, and antigen test positivity, alongside an unbiased head-to-head comparison of six commercial antigen tests." The authors thoroughly discuss their methods, including acquisition and use of study samples, use of qRT-PCR, choosing of rapid antigen tests, development of viral growth assays, and use of statistical analysis. After presenting their results and discussing them in detail, the authors conclude that their "data support the judicious use of LFDs for rapid antigen detection: not to replace PCR testing, but to supplement current testing capacity and rapidly identify infected individuals in situations in which they would otherwise go undetected," and that the benefits of careful LFD use "outweigh the risk of missing positive cases."
Posted on September 14, 2021
By LabLynx
Journal articles
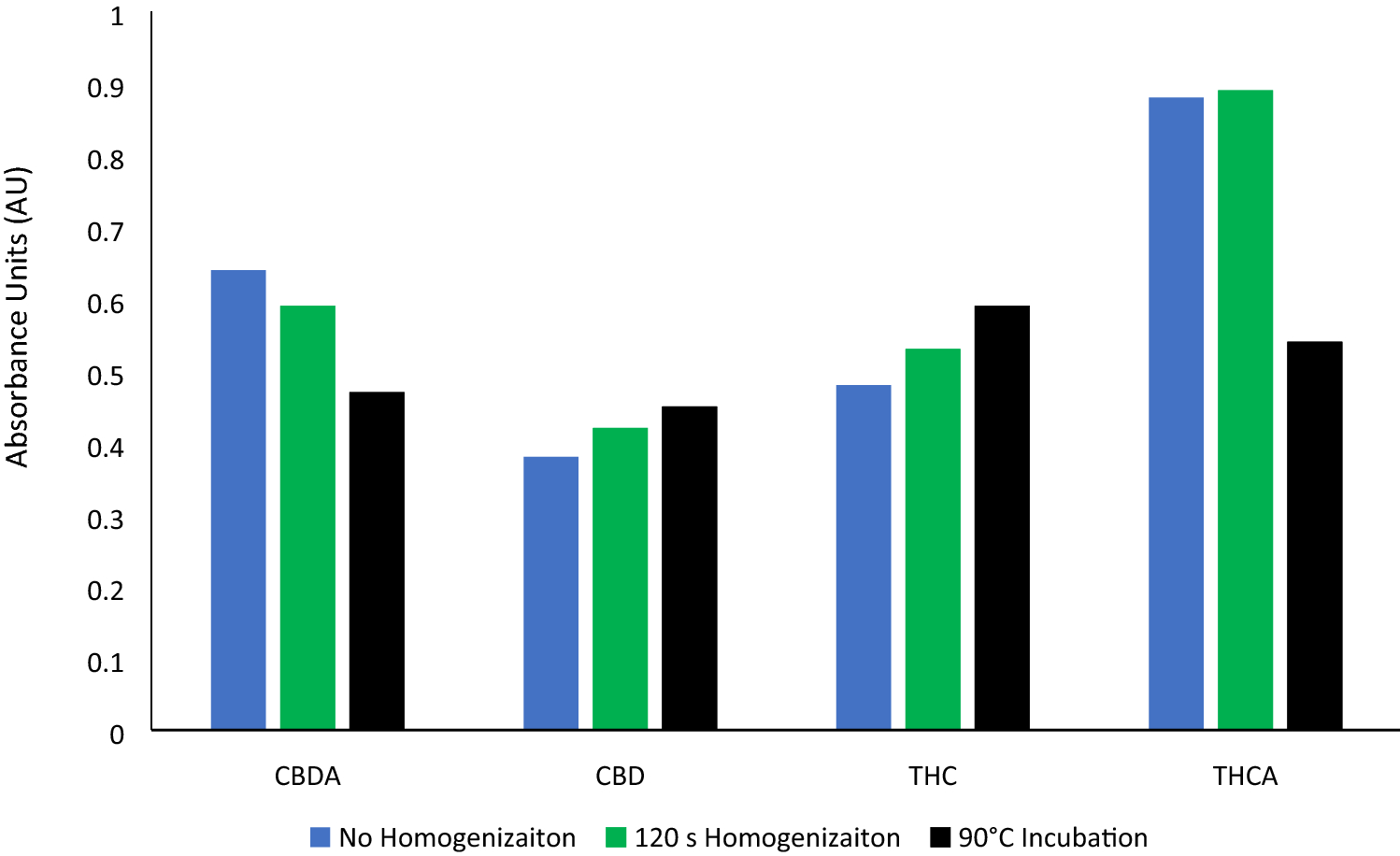
Analysis of
Cannabis and its constituents is seemingly always in a state of improvement and revision as we continue to learn more about the plant. In this 2021 paper published in
SN Applied Sciences, Morehouse
et al. propose another improvement to cannabinoid molecule concentration determinations for the purposes of differentiating hemp from marijuana and other potency testing measures. In their paper, the authors turn to an “active grinding media" using a bead mill for homogenizing the plant and improving cannabinoid recovery for testing. After explaining their methodology, presenting their results, and discussing their findings, the authors conclude that their method results in "increased cannabinoid recovery when compared to larger particle size homogenates, and no alterations in the carboxylation profiles of CBDA or THCA during processing." This proves to be a boon to those who need "to maintain the highest of potency and purity standards when moving forward with their molecular analysis."
Posted on September 7, 2021
By LabLynx
Journal articles
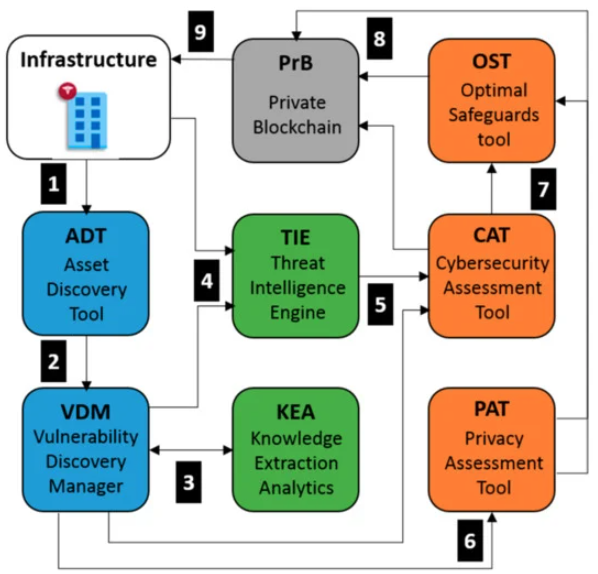
In this 2021 paper published in the journal
Applied Sciences, Jofre
et al. discuss the cybersecurity and privacy requirements associated with point-of-care (POC) and health information systems in the field of healthcare. In particular, the authors "propose a use-case approach to assess specifications of cybersecurity and privacy requirements of POC systems in a structured and self-contained form." After introducing the concept of cybersecurity in POC and other medical devices, they discuss their use case in the scope of the European Union's Secure and Private Health Data Exchange (CUREX) software platform for delivering better trust and security to applications in the healthcare domain. They provide in-depth details about their use case, its technical development, and its implementation in CUREX. They then discuss their results, concluding that as cybersecurity and privacy protection requirements continue to be implemented in healthcare, validated use cases such as their own will be vital for healthcare facilities adopting POC and related technologies, as they will further ensure "the highest quality for what is actually being delivered on the ground or showing promise in terms of developments in the pipeline."
Posted on August 31, 2021
By LabLynx
Journal articles
In this brief commentary article published in
Health Services Research and Managerial Epidemiology, Matthews and Proctor of the University of Central Florida argue that simply building out public health informatics infrastructure isn't enough to ensure better public health data and improved health outcomes in the populace. The duo notes that when considering the build out of such technologies, it's vital the implementing organization(s) ask "who are we building this product for, and do we have the right information to back up our theories on implementation and use?” Improved technology skills within the public health workforce aren't enough, they argue, and important human factors research is being neglected when planning for systems implementations. After discussing the importance of health factors research in public health practice and what it should look like, they conclude with four critical recommendations for new and existing public health informatics implementations, including stakeholder collaboration, theoretically informed and validated data collection, proper documentation, and malleable but well-informed technology requirements statements for the future.
Posted on August 24, 2021
By LabLynx
Journal articles
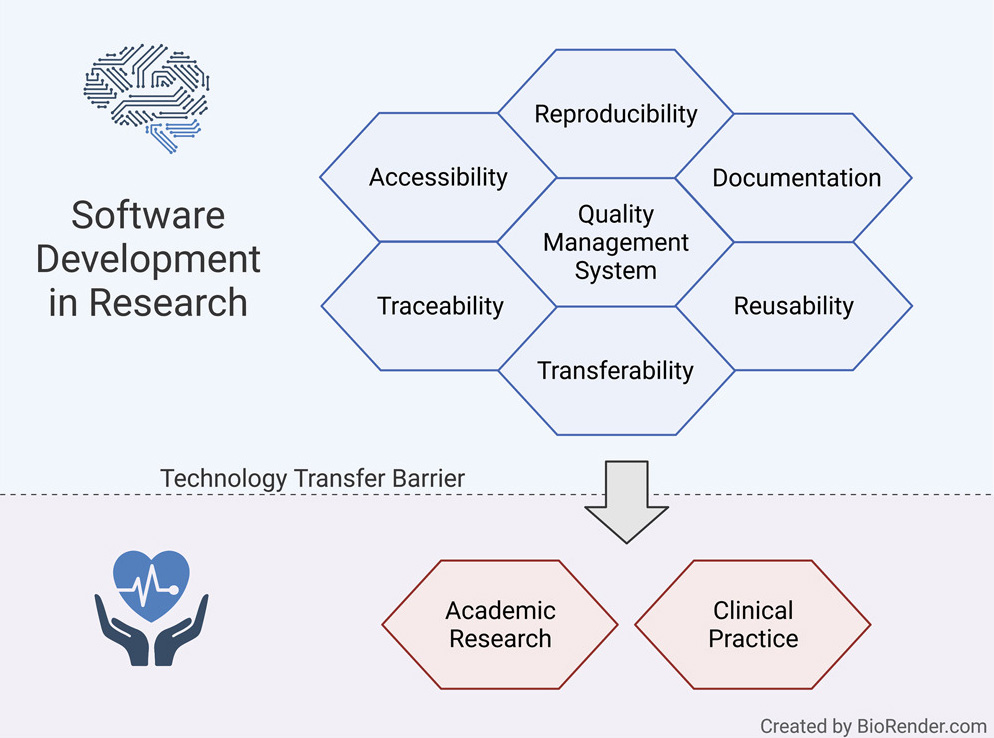
In this 2021 perspectives article published in the journal
iScience, Hauschild
et al. of Philipps University of Marburg lay out what they believe to be the first guidelines for an academic organization needing to incorporate a manageable and approachable quality management system (QMS) to support academic research, particularly that involving software developed in-house. Noting the challenges of academic scientific software development in health informatics and bioinformatics environments, the authors propose flexible quality management mechanisms as a means to "facilitate technology transfer as much as possible while keeping the overhead for researchers and developers in a well manageable range." After presenting the foundational guidelines for academic organizations to implement to not only promote reproducibility and reusability, but also support FAIR principles, the authors conclude that their guidelines effectively lower "the hurdle for research organizations to set up quality management" and " significantly facilitate reproducibility and reusability of scientific software and speed up technology transfer in a controlled and predictable way."
Posted on August 16, 2021
By LabLynx
Journal articles
Clinical informatics continues to be a vital component for helping to ensure quality clinical outcomes for patients. But what of those who must learn about clinical informatics and what it entails? What of its core competencies for learning? Davies
et al. explore this topic in the scope of the United Kingdom and the NHS, noting that no national core competency framework for clinical informatics exists in that country. The authors provide an introduction to clinical informatics and a background review of building their Core Competencies Project in an attempt to develop a U.K.-based competency framework. Their methods—heavily leaning on interviews and a survey—are then described, along with the resulting final competency framework. After a discussion of their results and their limitations, the authors conclude that their mixed-methods approach allowed them to "take a systematic and structured approach to co-designing the framework with no major issues," adding that the "combination of qualitative and quantitative methods contributed to the robustness of the final output." However, the application of the framework still requires professional considerations and attentiveness to the needs of all stakeholders to ensure it's not too unwieldy, which can lead to knowledge "burnout."
Posted on August 10, 2021
By LabLynx
Journal articles
This 2021 article published in the journal
Practical Laboratory Medicine examines laboratory testing in the scope of quality management and laboratory stewardship programs and the long-term benefits they bring to an organization. White
et al. first discuss the evolution of quality in the laboratory and then introduce the concept of "laboratory stewardship" and its ability to improve patient care. The rest of the work focuses on implementing such a program and presenting a hypothetical case study to show how a laboratory stewardship program can have a practical impact on a healthcare system. The authors conclude that a well-implemented stewardship program "presents a valuable opportunity for laboratory professionals to engage with clinical colleagues and drive change." They also distill their work down to five key elements required for making such programs have maximum impact: "1) a clear vision and organizational alignment; 2) appropriate skills for program execution and management; 3) resources to support the program; 4) incentives to motivate participation; and, 5) a plan of action that articulates program objectives and metrics."
 In this 2021 journal article published in Data Science Journal, Damerow et al. emphasize that while sample-based research is critical to a wide range of ecosystem sciences, the increasingly multidisciplinary approach to those sciences requires a better, more coordinated practice of sample identification and data management. "While there are widely adopted conventions within certain domains to describe sample data," they say, "these have gaps when applied in a multidisciplinary context." With this paper, the authors present a more practical approach to sample identification and management that takes into account the multidisciplinary requirements of ecosystems research. After a brief review of literature and existing sample identification methods, guidance, and standards, they describe their pilot program for standardizing sample metadata and propose several benefits to the program. They conclude that "user-friendly guidance and sample metadata templates are an essential step in promoting standard practices that make data publishing, integration, and reuse easier," though proper training, legacy data management tools, and information management systems are also important components towards those goals.
In this 2021 journal article published in Data Science Journal, Damerow et al. emphasize that while sample-based research is critical to a wide range of ecosystem sciences, the increasingly multidisciplinary approach to those sciences requires a better, more coordinated practice of sample identification and data management. "While there are widely adopted conventions within certain domains to describe sample data," they say, "these have gaps when applied in a multidisciplinary context." With this paper, the authors present a more practical approach to sample identification and management that takes into account the multidisciplinary requirements of ecosystems research. After a brief review of literature and existing sample identification methods, guidance, and standards, they describe their pilot program for standardizing sample metadata and propose several benefits to the program. They conclude that "user-friendly guidance and sample metadata templates are an essential step in promoting standard practices that make data publishing, integration, and reuse easier," though proper training, legacy data management tools, and information management systems are also important components towards those goals.
 The implementation of digital pathology workflows has seen an uptick in interest in recent years, though as Fraggetta et al. point out in this 2021 journal article, only a minority of pathology laboratories have fully embraced these workflows. Wanting to increase the adoption rate of digital pathology, the authors present their experiences with digital pathology and focus on four critical considerations to improve adoption rates. After presenting a brief introduction to digital pathology, the authors discuss various aspects of involvement, optimization, and automation required to get digital pathology projects initially started. They then commit a significant portion of their paper to discussing the quality control program that must be implemented as part of a digital pathology workflow. They also address whole slide imaging and its validation, as well as retention policies, results evaluation, and the necessary maintenance of workflows after implementation. They conclude with 10 basic principles (classified as recommendations or suggestions) to address when transitioning from "a classic, 'analog' to a completely digital workflow," adding that " the present document represents a practical, handy reference for the correct implementation of a digital workflow in Europe."
The implementation of digital pathology workflows has seen an uptick in interest in recent years, though as Fraggetta et al. point out in this 2021 journal article, only a minority of pathology laboratories have fully embraced these workflows. Wanting to increase the adoption rate of digital pathology, the authors present their experiences with digital pathology and focus on four critical considerations to improve adoption rates. After presenting a brief introduction to digital pathology, the authors discuss various aspects of involvement, optimization, and automation required to get digital pathology projects initially started. They then commit a significant portion of their paper to discussing the quality control program that must be implemented as part of a digital pathology workflow. They also address whole slide imaging and its validation, as well as retention policies, results evaluation, and the necessary maintenance of workflows after implementation. They conclude with 10 basic principles (classified as recommendations or suggestions) to address when transitioning from "a classic, 'analog' to a completely digital workflow," adding that " the present document represents a practical, handy reference for the correct implementation of a digital workflow in Europe."
 Veteran laboratory automation/computing professional Joe Liscouski is at it again, this time releasing a perspective piece that takes elements from more than 15 years of writing and presentation, painting a nuanced approach to planning for the use of computer systems in the laboratory. In particular, this November 2021 work continues to expand on the importance of laboratory systems engineering in the laboratory of the future. After providing a full introduction, Liscouski examines both the past and present of laboratory computing and how the automation aspects of that computing affects laboratory personnel. He then goes on to espouse the benefits of a more industry-wide approach to addressing the technological and educational needs of laboratories of all types, particularly in regard to how standardization plays an important role. He then addresses laboratory work itself, how automation can move that work forward, and how to effectively apply that automation to the laboratory. Finally, Liscouski closes by emphasizing the importance of a "center for laboratory systems engineering" to help centralize the efforts mentioned in the guide. A sizeable appendix is included, providing more historical perspective to the work and its conclusions.
Veteran laboratory automation/computing professional Joe Liscouski is at it again, this time releasing a perspective piece that takes elements from more than 15 years of writing and presentation, painting a nuanced approach to planning for the use of computer systems in the laboratory. In particular, this November 2021 work continues to expand on the importance of laboratory systems engineering in the laboratory of the future. After providing a full introduction, Liscouski examines both the past and present of laboratory computing and how the automation aspects of that computing affects laboratory personnel. He then goes on to espouse the benefits of a more industry-wide approach to addressing the technological and educational needs of laboratories of all types, particularly in regard to how standardization plays an important role. He then addresses laboratory work itself, how automation can move that work forward, and how to effectively apply that automation to the laboratory. Finally, Liscouski closes by emphasizing the importance of a "center for laboratory systems engineering" to help centralize the efforts mentioned in the guide. A sizeable appendix is included, providing more historical perspective to the work and its conclusions.
 In this 2021 paper published in Frontiers in Plant Science, Feder et al. present the analytical results of Cannabis inflorescences that have been pollinated and fertilized, in an attempt to show that fertilization can affect phytocannabinoid accumulation, among other goals. Noting that growers have recently shifted to using seeds, which can despite feminization reveal to be male seeds five to ten percent of the time, the authors note that this type of research—rarely conducted—is particularly critical to answering questions about how pollination effects phytocannabinoid and terpenoid expression. After providing background information and reviewing materials and methods for their analyses, the authors discuss the results, noting in particular that "phytocannabinoid quantity predominately decreases after fertilization," terpenoid quantity can vary based upon the type of female plant, and that "individual terpenoid concentrations are differentially affected by fertilization." After further discussion, they conclude not only the three prior-mentioned findings, but they also by extension suggest that their findings indicate the "functional roles" of phytocannabinoids and terpenoids in Cannabis's plant life cycle.
In this 2021 paper published in Frontiers in Plant Science, Feder et al. present the analytical results of Cannabis inflorescences that have been pollinated and fertilized, in an attempt to show that fertilization can affect phytocannabinoid accumulation, among other goals. Noting that growers have recently shifted to using seeds, which can despite feminization reveal to be male seeds five to ten percent of the time, the authors note that this type of research—rarely conducted—is particularly critical to answering questions about how pollination effects phytocannabinoid and terpenoid expression. After providing background information and reviewing materials and methods for their analyses, the authors discuss the results, noting in particular that "phytocannabinoid quantity predominately decreases after fertilization," terpenoid quantity can vary based upon the type of female plant, and that "individual terpenoid concentrations are differentially affected by fertilization." After further discussion, they conclude not only the three prior-mentioned findings, but they also by extension suggest that their findings indicate the "functional roles" of phytocannabinoids and terpenoids in Cannabis's plant life cycle.
 In this 2021 journal article published in Journal of Medical Biochemistry, Arifin and Yusof of Universiti Kebangsaan Malaysia examine the error factors that come with using a laboratory information system (LIS) and propose the "total testing process for laboratory information systems" (TTP-LIS). This process leans on a variety of existing frameworks and lean quality improvement methods to meet the authors' needs and is applied to two large hospitals in Malaysia. After examining human, technology, and organizational factors, the authors discuss their findings, noting that their "findings showed the practicality of the TTP-LIS framework as an evaluation tool in identifying errors and their causal factors. The use of lean tools—namely, VSM, A3, and 5 Why—enabled us to analyze and visualize the root cause of problems in an objective and structured manner. " Those root causes were able to be categorized in three ways: "as a latent failure in system development, as poor error management, and as unsatisfactory lab testing processes and LIS use."
In this 2021 journal article published in Journal of Medical Biochemistry, Arifin and Yusof of Universiti Kebangsaan Malaysia examine the error factors that come with using a laboratory information system (LIS) and propose the "total testing process for laboratory information systems" (TTP-LIS). This process leans on a variety of existing frameworks and lean quality improvement methods to meet the authors' needs and is applied to two large hospitals in Malaysia. After examining human, technology, and organizational factors, the authors discuss their findings, noting that their "findings showed the practicality of the TTP-LIS framework as an evaluation tool in identifying errors and their causal factors. The use of lean tools—namely, VSM, A3, and 5 Why—enabled us to analyze and visualize the root cause of problems in an objective and structured manner. " Those root causes were able to be categorized in three ways: "as a latent failure in system development, as poor error management, and as unsatisfactory lab testing processes and LIS use."
 When it comes to analyzing the Cannabis plant and its constituents, terpenes are one such component that hasn't been well studied until recent years. But as Sommano et al. note in their 2020 paper published in Molecules, "a growing number of industries have shown interest in adding either cannabis terpenes or botanically-derived terpenes to their CBD oils and edibles." This has resulted in demand for further study of terpenes and how they interact with other constituents. The authors present a broad look at terpenes in their paper. examining the taxonomy and localization of terpenes from Cannabis plants, their biosynthesis, and their prevalence in certain chemovars. They close by briefly discussing separation of terpenes, and concluding that "terpene profiles not only embody the characteristics of cannabis genotypes, but their entourage effect with cannabinoids could enhance their medicinal functionality."
When it comes to analyzing the Cannabis plant and its constituents, terpenes are one such component that hasn't been well studied until recent years. But as Sommano et al. note in their 2020 paper published in Molecules, "a growing number of industries have shown interest in adding either cannabis terpenes or botanically-derived terpenes to their CBD oils and edibles." This has resulted in demand for further study of terpenes and how they interact with other constituents. The authors present a broad look at terpenes in their paper. examining the taxonomy and localization of terpenes from Cannabis plants, their biosynthesis, and their prevalence in certain chemovars. They close by briefly discussing separation of terpenes, and concluding that "terpene profiles not only embody the characteristics of cannabis genotypes, but their entourage effect with cannabinoids could enhance their medicinal functionality."
 In this 2021 article published in the journal Diagnostics, Mrazek et al. of the Paracelsus Medical University provide a review of the available demand management (DM) strategies available to laboratorians and clinicians for avoiding inappropriate utilization of specific laboratory diagnostics. The authors provide 10 potential strategies toward that goal, including the likes of more practical order entry alerts, better utilization of the laboratory information system (LIS), outdated test removal, relevant test addition, and artificial intelligence (AI) algorithms, to name a few. However, whatever methods are implemented, the authors note that it's vital for demand management strategies "to be adapted to local settings." Additionally, given that many of the strategies may be unduly time-consuming, they "believe that AI solutions are the next logical step, aiding in the development as well as improvement of DM strategies, as they could help to manage large data sets." However, even AI has its caveats, as "a tool of assistance," they add.
In this 2021 article published in the journal Diagnostics, Mrazek et al. of the Paracelsus Medical University provide a review of the available demand management (DM) strategies available to laboratorians and clinicians for avoiding inappropriate utilization of specific laboratory diagnostics. The authors provide 10 potential strategies toward that goal, including the likes of more practical order entry alerts, better utilization of the laboratory information system (LIS), outdated test removal, relevant test addition, and artificial intelligence (AI) algorithms, to name a few. However, whatever methods are implemented, the authors note that it's vital for demand management strategies "to be adapted to local settings." Additionally, given that many of the strategies may be unduly time-consuming, they "believe that AI solutions are the next logical step, aiding in the development as well as improvement of DM strategies, as they could help to manage large data sets." However, even AI has its caveats, as "a tool of assistance," they add.
 As laboratories continue to swim in an increasing amount of data and attempt to streamline operations, thoughts of turning to cloud-based data management grow. And while cloud-based applications can help, challenges remain with security, privacy, and real-time access. Blockchain-based systems alone can improve on some of those challenges, but blockchain isn't scalable. As such, Ismail et al. discuss at length in this 2021 paper the benefits of integrating blockchain with cloud-computing systems, as well as the remaining challenges of optimizing such an integration. After presenting existing research and discussing the basics of cloud computing and blockchain, the authors discuss the integration of the two in both encapsulated- and non-encapsulated architectures, as well as how they can be applied to healthcare systems. They close by addressing the remaining challenges of an integrated system, including the energy costs of running such a system. They conclude that while "the integration of cloud and blockchain for healthcare is promising to cope with the shortcomings of these individual technologies ... further research is still required to enhance the existing architecture to make it more scalable and energy-efficient with inter-cloud communication support."
As laboratories continue to swim in an increasing amount of data and attempt to streamline operations, thoughts of turning to cloud-based data management grow. And while cloud-based applications can help, challenges remain with security, privacy, and real-time access. Blockchain-based systems alone can improve on some of those challenges, but blockchain isn't scalable. As such, Ismail et al. discuss at length in this 2021 paper the benefits of integrating blockchain with cloud-computing systems, as well as the remaining challenges of optimizing such an integration. After presenting existing research and discussing the basics of cloud computing and blockchain, the authors discuss the integration of the two in both encapsulated- and non-encapsulated architectures, as well as how they can be applied to healthcare systems. They close by addressing the remaining challenges of an integrated system, including the energy costs of running such a system. They conclude that while "the integration of cloud and blockchain for healthcare is promising to cope with the shortcomings of these individual technologies ... further research is still required to enhance the existing architecture to make it more scalable and energy-efficient with inter-cloud communication support."
 There are numerous laboratory information management systems (LIMS) on the market, but in reality, how many are finely tuned to handle the safety requirements of a biosafety laboratory? At least from the standpoint of Sun et al. of the Institute of Microbiology Chinese Academy of Sciences, few if any meet those needs. As such, the authors, in this 2021 paper published in the Journal of Biosafety and Biosecurity, commence with laying out the unique add-on requirements a standard LIMS would require in order to meet the needs of most biosafety lab. Noting in their introduction "a strange situation, one where biosafety information itself is highly digitalized, yet there is no centralized system designed to better organize that electronic information and enable laboratorians to use it appropriately," the authors then describe what is required to remedy that situation. They note that biosafety and efficiency are tied to four information management aspects of the biosafety lab: project management, personnel administration, experimental material management, and equipment management. The authors then discuss how these areas of management would need to be fortified in a modern LIMS, as well as addressing other areas such as balancing flexibility and complexity and addressing data security.
There are numerous laboratory information management systems (LIMS) on the market, but in reality, how many are finely tuned to handle the safety requirements of a biosafety laboratory? At least from the standpoint of Sun et al. of the Institute of Microbiology Chinese Academy of Sciences, few if any meet those needs. As such, the authors, in this 2021 paper published in the Journal of Biosafety and Biosecurity, commence with laying out the unique add-on requirements a standard LIMS would require in order to meet the needs of most biosafety lab. Noting in their introduction "a strange situation, one where biosafety information itself is highly digitalized, yet there is no centralized system designed to better organize that electronic information and enable laboratorians to use it appropriately," the authors then describe what is required to remedy that situation. They note that biosafety and efficiency are tied to four information management aspects of the biosafety lab: project management, personnel administration, experimental material management, and equipment management. The authors then discuss how these areas of management would need to be fortified in a modern LIMS, as well as addressing other areas such as balancing flexibility and complexity and addressing data security.
 In this 2021 journal article published in the Journal of Pathology Informatics, Starks et al. of the University of Iowa Hospitals and Clinics present their case for the value of autoverification data evaluation despite the inherent challenges of extracting and analyzing data related to autoverification rules from informatics system. Noting that most autoverification rules were housed in their middleware solution and (and not the LIS, which would require additional fields currently not present), they examined instrument- and middleware-generated flags generated from "complete blood count (CBC) with white blood cell (WBC) count differential (Diff) and the 'a la carte' ordering of individual CBC components." Their analysis resulted in two significant changes to their autoverification rules related to those tests, resulting in "improved efficiency and lower rerun rates." However, these insights did come at some investment in time and resources, requiring third-party data retrieval methods and "extensive cleanup and formatting" of the data to actually identify flagged specimens. The authors conclude that these downsides highlight "opportunities for improvement in software tools that allow for more rapid and routine evaluation of autoverification parameters."
In this 2021 journal article published in the Journal of Pathology Informatics, Starks et al. of the University of Iowa Hospitals and Clinics present their case for the value of autoverification data evaluation despite the inherent challenges of extracting and analyzing data related to autoverification rules from informatics system. Noting that most autoverification rules were housed in their middleware solution and (and not the LIS, which would require additional fields currently not present), they examined instrument- and middleware-generated flags generated from "complete blood count (CBC) with white blood cell (WBC) count differential (Diff) and the 'a la carte' ordering of individual CBC components." Their analysis resulted in two significant changes to their autoverification rules related to those tests, resulting in "improved efficiency and lower rerun rates." However, these insights did come at some investment in time and resources, requiring third-party data retrieval methods and "extensive cleanup and formatting" of the data to actually identify flagged specimens. The authors conclude that these downsides highlight "opportunities for improvement in software tools that allow for more rapid and routine evaluation of autoverification parameters."
 Managing cybersecurity within an organization can be challenging, more so when the in-house knowledge and management drive concerning cybersecurity is lacking. But does it have to be so complicated? What tools exist to help organizations sort through and implement proper safeguards for their cyber infrastructure, and in particular their associated privacy-specific data? In this 2021 paper published in Sensors, Gonzalez-Granadillo et al. propose a privacy risk management toolkit called AMBIENT (Automated Cyber and Privacy Risk Management Toolkit) that "not only assesses cyber and privacy risks in a thorough and automated manner, but it also offers decision-support capabilities to recommend optimal safeguards using the well-known repository of the Center for Internet Security (CIS) Controls." After presenting related work, describing AMBIENT, and showing the results of a demo, the author conclude that "security managers can use AMBIENT’s results as a guide in their decision-making process to define appropriate security policies and strategies that keep risk scores within acceptable levels."
Managing cybersecurity within an organization can be challenging, more so when the in-house knowledge and management drive concerning cybersecurity is lacking. But does it have to be so complicated? What tools exist to help organizations sort through and implement proper safeguards for their cyber infrastructure, and in particular their associated privacy-specific data? In this 2021 paper published in Sensors, Gonzalez-Granadillo et al. propose a privacy risk management toolkit called AMBIENT (Automated Cyber and Privacy Risk Management Toolkit) that "not only assesses cyber and privacy risks in a thorough and automated manner, but it also offers decision-support capabilities to recommend optimal safeguards using the well-known repository of the Center for Internet Security (CIS) Controls." After presenting related work, describing AMBIENT, and showing the results of a demo, the author conclude that "security managers can use AMBIENT’s results as a guide in their decision-making process to define appropriate security policies and strategies that keep risk scores within acceptable levels."
 In this 2021 article published in Forensic Science International Reports, Viviers et al. present their findings of a wide assessment of heavy metals content in South African cannabis products. Noting a dearth of such research in the South African market, the authors acquired 310 samples (representing seven different sample types) from "cultivators of plants, producers of products, importers, resellers, and pharmaceutical manufacturers" and had them analyzed for their various residues. The authors turned to inductively coupled plasma mass spectrometry (ICP-MS), the United States Pharmacopeia (USP) <232>/<233> and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q3D to guide their analyses. After a brief discussion of materials and methods, the authors present their results and conclusions. They found "among all residues analyzed, a Class 1 residue will be present in a third of all samples" (cadmium, lead, arsenic, and mercury), 15% of all samples failed heavy metal analysis "when compared against the USP <232>/<233> and ICH Q3D oral specification limit," and 44% failed when compared to the specified inhalation limits. The conclude that "when considering the results obtained, it is exceedingly necessary to have an appropriate quality control system in place, especially for heavy metal residues analysis in the current South African market. "
In this 2021 article published in Forensic Science International Reports, Viviers et al. present their findings of a wide assessment of heavy metals content in South African cannabis products. Noting a dearth of such research in the South African market, the authors acquired 310 samples (representing seven different sample types) from "cultivators of plants, producers of products, importers, resellers, and pharmaceutical manufacturers" and had them analyzed for their various residues. The authors turned to inductively coupled plasma mass spectrometry (ICP-MS), the United States Pharmacopeia (USP) <232>/<233> and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q3D to guide their analyses. After a brief discussion of materials and methods, the authors present their results and conclusions. They found "among all residues analyzed, a Class 1 residue will be present in a third of all samples" (cadmium, lead, arsenic, and mercury), 15% of all samples failed heavy metal analysis "when compared against the USP <232>/<233> and ICH Q3D oral specification limit," and 44% failed when compared to the specified inhalation limits. The conclude that "when considering the results obtained, it is exceedingly necessary to have an appropriate quality control system in place, especially for heavy metal residues analysis in the current South African market. "
 Using computer-based resources to find and retrieve healthcare information is increasingly common, whether it's the healthcare professional or a patient. But "search can be challenging, particularly for health informatics tasks that utilize large and complex document sets," notes Demelo and Sedig in their 2021 paper published in Information. This led the authors to examine the current state of healthcare informatics and develop an ontology-based search interface called ONTSI. Based on insights from previously published research on health informatics information retrieval, the authors discuss the criteria, design elements, and strategies they used to develop ONTSI. After explaining the results of their work, via a use case, the authors conclude that their tool "ONTSI supplies a generalized interface that supports users’ ability to plug-and-play their provided document sets and an ontology file as a mediating resource within the interface when performing their health informatics search tasks."
Using computer-based resources to find and retrieve healthcare information is increasingly common, whether it's the healthcare professional or a patient. But "search can be challenging, particularly for health informatics tasks that utilize large and complex document sets," notes Demelo and Sedig in their 2021 paper published in Information. This led the authors to examine the current state of healthcare informatics and develop an ontology-based search interface called ONTSI. Based on insights from previously published research on health informatics information retrieval, the authors discuss the criteria, design elements, and strategies they used to develop ONTSI. After explaining the results of their work, via a use case, the authors conclude that their tool "ONTSI supplies a generalized interface that supports users’ ability to plug-and-play their provided document sets and an ontology file as a mediating resource within the interface when performing their health informatics search tasks."
 In this 2021 article published in the journal The Lancet Microbe, Pickering et al. present their findings concerning the comparison of antigen lateral flow devices (LFDs) for COVID-19 and virus infectivity, noting their study is "the largest to date assessing the correlation between [cycle threshold] values, quantitative culture of infectious virus, and antigen test positivity, alongside an unbiased head-to-head comparison of six commercial antigen tests." The authors thoroughly discuss their methods, including acquisition and use of study samples, use of qRT-PCR, choosing of rapid antigen tests, development of viral growth assays, and use of statistical analysis. After presenting their results and discussing them in detail, the authors conclude that their "data support the judicious use of LFDs for rapid antigen detection: not to replace PCR testing, but to supplement current testing capacity and rapidly identify infected individuals in situations in which they would otherwise go undetected," and that the benefits of careful LFD use "outweigh the risk of missing positive cases."
In this 2021 article published in the journal The Lancet Microbe, Pickering et al. present their findings concerning the comparison of antigen lateral flow devices (LFDs) for COVID-19 and virus infectivity, noting their study is "the largest to date assessing the correlation between [cycle threshold] values, quantitative culture of infectious virus, and antigen test positivity, alongside an unbiased head-to-head comparison of six commercial antigen tests." The authors thoroughly discuss their methods, including acquisition and use of study samples, use of qRT-PCR, choosing of rapid antigen tests, development of viral growth assays, and use of statistical analysis. After presenting their results and discussing them in detail, the authors conclude that their "data support the judicious use of LFDs for rapid antigen detection: not to replace PCR testing, but to supplement current testing capacity and rapidly identify infected individuals in situations in which they would otherwise go undetected," and that the benefits of careful LFD use "outweigh the risk of missing positive cases."
 Analysis of Cannabis and its constituents is seemingly always in a state of improvement and revision as we continue to learn more about the plant. In this 2021 paper published in SN Applied Sciences, Morehouse et al. propose another improvement to cannabinoid molecule concentration determinations for the purposes of differentiating hemp from marijuana and other potency testing measures. In their paper, the authors turn to an “active grinding media" using a bead mill for homogenizing the plant and improving cannabinoid recovery for testing. After explaining their methodology, presenting their results, and discussing their findings, the authors conclude that their method results in "increased cannabinoid recovery when compared to larger particle size homogenates, and no alterations in the carboxylation profiles of CBDA or THCA during processing." This proves to be a boon to those who need "to maintain the highest of potency and purity standards when moving forward with their molecular analysis."
Analysis of Cannabis and its constituents is seemingly always in a state of improvement and revision as we continue to learn more about the plant. In this 2021 paper published in SN Applied Sciences, Morehouse et al. propose another improvement to cannabinoid molecule concentration determinations for the purposes of differentiating hemp from marijuana and other potency testing measures. In their paper, the authors turn to an “active grinding media" using a bead mill for homogenizing the plant and improving cannabinoid recovery for testing. After explaining their methodology, presenting their results, and discussing their findings, the authors conclude that their method results in "increased cannabinoid recovery when compared to larger particle size homogenates, and no alterations in the carboxylation profiles of CBDA or THCA during processing." This proves to be a boon to those who need "to maintain the highest of potency and purity standards when moving forward with their molecular analysis."
 In this 2021 paper published in the journal Applied Sciences, Jofre et al. discuss the cybersecurity and privacy requirements associated with point-of-care (POC) and health information systems in the field of healthcare. In particular, the authors "propose a use-case approach to assess specifications of cybersecurity and privacy requirements of POC systems in a structured and self-contained form." After introducing the concept of cybersecurity in POC and other medical devices, they discuss their use case in the scope of the European Union's Secure and Private Health Data Exchange (CUREX) software platform for delivering better trust and security to applications in the healthcare domain. They provide in-depth details about their use case, its technical development, and its implementation in CUREX. They then discuss their results, concluding that as cybersecurity and privacy protection requirements continue to be implemented in healthcare, validated use cases such as their own will be vital for healthcare facilities adopting POC and related technologies, as they will further ensure "the highest quality for what is actually being delivered on the ground or showing promise in terms of developments in the pipeline."
In this 2021 paper published in the journal Applied Sciences, Jofre et al. discuss the cybersecurity and privacy requirements associated with point-of-care (POC) and health information systems in the field of healthcare. In particular, the authors "propose a use-case approach to assess specifications of cybersecurity and privacy requirements of POC systems in a structured and self-contained form." After introducing the concept of cybersecurity in POC and other medical devices, they discuss their use case in the scope of the European Union's Secure and Private Health Data Exchange (CUREX) software platform for delivering better trust and security to applications in the healthcare domain. They provide in-depth details about their use case, its technical development, and its implementation in CUREX. They then discuss their results, concluding that as cybersecurity and privacy protection requirements continue to be implemented in healthcare, validated use cases such as their own will be vital for healthcare facilities adopting POC and related technologies, as they will further ensure "the highest quality for what is actually being delivered on the ground or showing promise in terms of developments in the pipeline."
 In this 2021 perspectives article published in the journal iScience, Hauschild et al. of Philipps University of Marburg lay out what they believe to be the first guidelines for an academic organization needing to incorporate a manageable and approachable quality management system (QMS) to support academic research, particularly that involving software developed in-house. Noting the challenges of academic scientific software development in health informatics and bioinformatics environments, the authors propose flexible quality management mechanisms as a means to "facilitate technology transfer as much as possible while keeping the overhead for researchers and developers in a well manageable range." After presenting the foundational guidelines for academic organizations to implement to not only promote reproducibility and reusability, but also support FAIR principles, the authors conclude that their guidelines effectively lower "the hurdle for research organizations to set up quality management" and " significantly facilitate reproducibility and reusability of scientific software and speed up technology transfer in a controlled and predictable way."
In this 2021 perspectives article published in the journal iScience, Hauschild et al. of Philipps University of Marburg lay out what they believe to be the first guidelines for an academic organization needing to incorporate a manageable and approachable quality management system (QMS) to support academic research, particularly that involving software developed in-house. Noting the challenges of academic scientific software development in health informatics and bioinformatics environments, the authors propose flexible quality management mechanisms as a means to "facilitate technology transfer as much as possible while keeping the overhead for researchers and developers in a well manageable range." After presenting the foundational guidelines for academic organizations to implement to not only promote reproducibility and reusability, but also support FAIR principles, the authors conclude that their guidelines effectively lower "the hurdle for research organizations to set up quality management" and " significantly facilitate reproducibility and reusability of scientific software and speed up technology transfer in a controlled and predictable way."
 Clinical informatics continues to be a vital component for helping to ensure quality clinical outcomes for patients. But what of those who must learn about clinical informatics and what it entails? What of its core competencies for learning? Davies et al. explore this topic in the scope of the United Kingdom and the NHS, noting that no national core competency framework for clinical informatics exists in that country. The authors provide an introduction to clinical informatics and a background review of building their Core Competencies Project in an attempt to develop a U.K.-based competency framework. Their methods—heavily leaning on interviews and a survey—are then described, along with the resulting final competency framework. After a discussion of their results and their limitations, the authors conclude that their mixed-methods approach allowed them to "take a systematic and structured approach to co-designing the framework with no major issues," adding that the "combination of qualitative and quantitative methods contributed to the robustness of the final output." However, the application of the framework still requires professional considerations and attentiveness to the needs of all stakeholders to ensure it's not too unwieldy, which can lead to knowledge "burnout."
Clinical informatics continues to be a vital component for helping to ensure quality clinical outcomes for patients. But what of those who must learn about clinical informatics and what it entails? What of its core competencies for learning? Davies et al. explore this topic in the scope of the United Kingdom and the NHS, noting that no national core competency framework for clinical informatics exists in that country. The authors provide an introduction to clinical informatics and a background review of building their Core Competencies Project in an attempt to develop a U.K.-based competency framework. Their methods—heavily leaning on interviews and a survey—are then described, along with the resulting final competency framework. After a discussion of their results and their limitations, the authors conclude that their mixed-methods approach allowed them to "take a systematic and structured approach to co-designing the framework with no major issues," adding that the "combination of qualitative and quantitative methods contributed to the robustness of the final output." However, the application of the framework still requires professional considerations and attentiveness to the needs of all stakeholders to ensure it's not too unwieldy, which can lead to knowledge "burnout."
 This 2021 article published in the journal Practical Laboratory Medicine examines laboratory testing in the scope of quality management and laboratory stewardship programs and the long-term benefits they bring to an organization. White et al. first discuss the evolution of quality in the laboratory and then introduce the concept of "laboratory stewardship" and its ability to improve patient care. The rest of the work focuses on implementing such a program and presenting a hypothetical case study to show how a laboratory stewardship program can have a practical impact on a healthcare system. The authors conclude that a well-implemented stewardship program "presents a valuable opportunity for laboratory professionals to engage with clinical colleagues and drive change." They also distill their work down to five key elements required for making such programs have maximum impact: "1) a clear vision and organizational alignment; 2) appropriate skills for program execution and management; 3) resources to support the program; 4) incentives to motivate participation; and, 5) a plan of action that articulates program objectives and metrics."
This 2021 article published in the journal Practical Laboratory Medicine examines laboratory testing in the scope of quality management and laboratory stewardship programs and the long-term benefits they bring to an organization. White et al. first discuss the evolution of quality in the laboratory and then introduce the concept of "laboratory stewardship" and its ability to improve patient care. The rest of the work focuses on implementing such a program and presenting a hypothetical case study to show how a laboratory stewardship program can have a practical impact on a healthcare system. The authors conclude that a well-implemented stewardship program "presents a valuable opportunity for laboratory professionals to engage with clinical colleagues and drive change." They also distill their work down to five key elements required for making such programs have maximum impact: "1) a clear vision and organizational alignment; 2) appropriate skills for program execution and management; 3) resources to support the program; 4) incentives to motivate participation; and, 5) a plan of action that articulates program objectives and metrics."
















